The Effect of Body Mass Index Changes between Two Consecutive Pregnancies on the Recurrence of Gestational Diabetes Mellitus in Japan
Article Information
Masako Dateki1, Seishi Furukawa1*, Syunichi Noda2, and Hiroshi Sameshima1
1Department of Obstetrics & Gynecology, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
2 Noda Obstetrics & Gynecology Clinic, Miyazaki, Japan
*Corresponding Author: Seishi Furukawa, Department of Obstetrics & Gynecology, Faculty of Medicine, University of Miyazaki 5200 Kihara-Kiyotake, Miyazaki, 889-1692, Japan
Received: 08 March 2020; Accepted: 16 March 2020; Published: 20 March 2020
Citation:
Masako Dateki, Seishi Furukawa, Syunichi Noda, and Hiroshi Sameshima. The Effect of Body Mass Index Changes between Two Consecutive Pregnancies on the Recurrence of Gestational Diabetes Mellitus in Japan. Journal of Women’s Health and Development 3 (2020): 056-064.
View / Download Pdf Share at FacebookAbstract
Aim: To identify the effect of interpregnancy pre-pregnancy body mass index changes (ΔBMI) on the recurrence of gestational diabetes mellitus (GDM).
Method: A cross sectional study was conducted comprising 183 cases diagnosed with GDM at least once in two consecutive pregnancies. Study cases were divided into three groups based on two consecutive glucose tolerance profiles that comprised normal glucose tolerance (NGT); GDM/GDM (n=45), GDM/NGT (n=33), and NGT/GDM (n=105). We compared ΔBMI among the groups. Study cases were then divided into subgroups on the basis of being below or above the median pre-pregnancy BMI at index pregnancy and ΔBMI was compared based on glucose tolerance profiles.
Results: The NGT/GDM group had the highest ΔBMI (1.01±2.06) among the 3 groups. The GDM/GDM group had a higher ΔBMI (0.52±1.59) compared with the GDM/NGT group (-0.41±1.50, p<0.01). The median pre-pregnancy BMI at index pregnancies was 21.2. In the GDM/NGT group, there was no difference in ΔBMI below or above the pre-pregnancy BMI of 21.2 (p=0.66). In the GDM/GDM group, there was no difference in ΔBMI below or above the pre-pregnancy BMI of 21.2 (p=0.97). In cases that fell below the pre-pregnancy BMI of 21.2, the GDM/NGT group was associated with a lower ΔBMI (-0.28±1.13) compared with the GDM/GDM group (0.51±1.23, p<0.05). In cases above the pre-pregnancy BMI of 21.2, there was no difference in ΔBMI between GDM/NGT (-0.64±2.04) and GDM/GDM groups (0.52±1.82, p=0.11).
Conclusion: Subtle changes in ΔBMI are associated with GDM recurrence, and reduced ΔBMI suppresses GDM recurrence in Japanese lean women.
Keywords
GDM; Recurrence; Lean; BMI, Weight gain; Interpregnancy
GDM articles GDM Research articles GDM review articles GDM PubMed articles GDM PubMed Central articles GDM 2023 articles GDM 2024 articles GDM Scopus articles GDM impact factor journals GDM Scopus journals GDM PubMed journals GDM medical journals GDM free journals GDM best journals GDM top journals GDM free medical journals GDM famous journals GDM Google Scholar indexed journals Recurrence articles Recurrence Research articles Recurrence review articles Recurrence PubMed articles Recurrence PubMed Central articles Recurrence 2023 articles Recurrence 2024 articles Recurrence Scopus articles Recurrence impact factor journals Recurrence Scopus journals Recurrence PubMed journals Recurrence medical journals Recurrence free journals Recurrence best journals Recurrence top journals Recurrence free medical journals Recurrence famous journals Recurrence Google Scholar indexed journals Lean articles Lean Research articles Lean review articles Lean PubMed articles Lean PubMed Central articles Lean 2023 articles Lean 2024 articles Lean Scopus articles Lean impact factor journals Lean Scopus journals Lean PubMed journals Lean medical journals Lean free journals Lean best journals Lean top journals Lean free medical journals Lean famous journals Lean Google Scholar indexed journals BMI articles BMI Research articles BMI review articles BMI PubMed articles BMI PubMed Central articles BMI 2023 articles BMI 2024 articles BMI Scopus articles BMI impact factor journals BMI Scopus journals BMI PubMed journals BMI medical journals BMI free journals BMI best journals BMI top journals BMI free medical journals BMI famous journals BMI Google Scholar indexed journals Weight gain articles Weight gain Research articles Weight gain review articles Weight gain PubMed articles Weight gain PubMed Central articles Weight gain 2023 articles Weight gain 2024 articles Weight gain Scopus articles Weight gain impact factor journals Weight gain Scopus journals Weight gain PubMed journals Weight gain medical journals Weight gain free journals Weight gain best journals Weight gain top journals Weight gain free medical journals Weight gain famous journals Weight gain Google Scholar indexed journals Interpregnancy articles Interpregnancy Research articles Interpregnancy review articles Interpregnancy PubMed articles Interpregnancy PubMed Central articles Interpregnancy 2023 articles Interpregnancy 2024 articles Interpregnancy Scopus articles Interpregnancy impact factor journals Interpregnancy Scopus journals Interpregnancy PubMed journals Interpregnancy medical journals Interpregnancy free journals Interpregnancy best journals Interpregnancy top journals Interpregnancy free medical journals Interpregnancy famous journals Interpregnancy Google Scholar indexed journals women articles women Research articles women review articles women PubMed articles women PubMed Central articles women 2023 articles women 2024 articles women Scopus articles women impact factor journals women Scopus journals women PubMed journals women medical journals women free journals women best journals women top journals women free medical journals women famous journals women Google Scholar indexed journals diabetes mellitus articles diabetes mellitus Research articles diabetes mellitus review articles diabetes mellitus PubMed articles diabetes mellitus PubMed Central articles diabetes mellitus 2023 articles diabetes mellitus 2024 articles diabetes mellitus Scopus articles diabetes mellitus impact factor journals diabetes mellitus Scopus journals diabetes mellitus PubMed journals diabetes mellitus medical journals diabetes mellitus free journals diabetes mellitus best journals diabetes mellitus top journals diabetes mellitus free medical journals diabetes mellitus famous journals diabetes mellitus Google Scholar indexed journals Characteristics articles Characteristics Research articles Characteristics review articles Characteristics PubMed articles Characteristics PubMed Central articles Characteristics 2023 articles Characteristics 2024 articles Characteristics Scopus articles Characteristics impact factor journals Characteristics Scopus journals Characteristics PubMed journals Characteristics medical journals Characteristics free journals Characteristics best journals Characteristics top journals Characteristics free medical journals Characteristics famous journals Characteristics Google Scholar indexed journals pre-pregnancy articles pre-pregnancy Research articles pre-pregnancy review articles pre-pregnancy PubMed articles pre-pregnancy PubMed Central articles pre-pregnancy 2023 articles pre-pregnancy 2024 articles pre-pregnancy Scopus articles pre-pregnancy impact factor journals pre-pregnancy Scopus journals pre-pregnancy PubMed journals pre-pregnancy medical journals pre-pregnancy free journals pre-pregnancy best journals pre-pregnancy top journals pre-pregnancy free medical journals pre-pregnancy famous journals pre-pregnancy Google Scholar indexed journals
Article Details
Introduction
Type 2 diabetes mellitus (type 2 DM) is characterized by high insulin resistance and decreased insulin secretion. The decrease in insulin secretion in type 2 DM is considered to be more advanced than before the onset [1, 2] and decreased insulin secretion has also been confirmed in gestational diabetes mellitus (GDM) [3]. Over half of women having GDM develop type 2 DM within the first decade after pregnancy with GDM [4]. Efforts to maintain insulin secretion and reduce insulin resistance may prevent or delay the progression of GDM to type 2 DM in women with a history of GDM.
Tips for preventing or delaying the progression of GDM to type 2 DM may relate to risk factors for GDM recurrence. Risk factors for recurrence of GDM are diverse, and are strongly correlated with insulin use during pregnancy, body mass index (BMI), prolific women, history of giving birth to heavy-for-dates infants, and weight gain between pregnancies [5]. In Japan, risk factors for recurrence of GDM include obesity, maternal age, prolific women, postpartum HbA1c, weight changes between pregnancies, and short pregnancy intervals [6]. Of these factors, one that can be realistically considered to prevent the recurrence of GDM is weight change between pregnancies. In fact, suppressing weight gain between two consecutive pregnancies had the effect of reducing GDM recurrence, in obese women in the United States [7]. It has also been reported that an increase in weight gain during the follow-up is associated with the progression of GDM to type 2 DMin women with a history GDM [8]. With respect to Japan, the prevalence of obesity in adult females is 3.2%, far lower than that in other developed countries [9]. Additionally, Japanese possess an ethnic characteristic that tends towards low insulin secretion ability [2, 3, 10]. It is therefore unclear how reducing weight gain between pregnancies might affect GDM recurrence in leaner Japanese women with low insulin secretion ability.
We therefore conducted a cross sectional study of women diagnosed with GDM in two consecutive pregnancies in an effort to evaluate the effect of body mass index changes between the two consecutive pregnancies on the recurrence of GDM. Our results provide practical data to prevent or delay the progression of GDM to type 2 DM in the Japanese population.
Methods
This study was undertaken retrospectively and approval (#2019 O-0575) was obtained from the constituted ethics committee of the University of Miyazaki. Medical charts of women who visited Noda Obstetrics & Gynecology Clinic from April 2012 to March 2017 and who were diagnosed with GDM at least once in two consecutive pregnancies were considered for this study. Noda Obstetrics & Gynecology Clinic is a private clinic located in the city of Miyakonojo, Miyazaki Prefecture and handles only low-risk pregnancies. The number of deliveries during this period in Noda Obstetrics & Gynecology Clinic was 3891.
During the study period, all pregnant women underwent measurement of random blood glucose levels in early-pregnancy (8~14 weeks of gestation) and mid-pregnancy (24~28 weeks of gestation). One hundred mg/dL of random blood glucose or more was regarded as positive for screening and a 75 g oral glucose tolerance test (OGTT) was then performed. Pregnant women who were not diagnosed with GDM in early-pregnancy were screened for GDM in mid-pregnancy. Additionally, if women had any risk factors for GDM such as obesity or history of GDM, a 75 g OGTT was performed. GDMis diagnosed if one or more of the following criteria is met in a 75g OGTT during pregnancy: 1: fasting plasma glucose level ≥92 mg/dL (5.1 mmol/L), 2: 1-hour value ≥180 mg/dL (10.0 mmol/L), 3: 2-hour value ≥153 mg/dL (8.5 mmol/L), according to diagnostic criteria of diabetes mellitus in Japan [11]. Pregnant women who did not meet the GDM criteria during pregnancy were regarded as having normal glucose tolerance (NGT).
Cases were divided into three groups based on two consecutive glucose tolerance profiles in two consecutive pregnancies, yielding GDM/GDM, GDM/NGT and NGT/GDM groups. The following maternal demographic data of the GDM/GDM, GDM/NGT and NGT/GDM groups were collected: maternal age at both pregnancies, parity (2 or more births) at index pregnancy, pre-pregnancy BMI at both pregnancies, baby birth weight at index pregnancy (g), and duration of breast feeding until subsequent pregnancy (months). We defined pre-pregnancy body mass index changes between two consecutive pregnancies as ΔBMI, and compared ΔBMI among the groups. Next, in accordance with the characteristics of the Japanese population, where the prevalence of obesity is extremely low compared with other developed countries [9], we evaluated changes in ΔBMI with respect to recurrence of GDM, taking into account BMI distribution before pregnancy in our cases. For that purpose, the median pre-pregnancy BMI at index pregnancies was examined. Each group was then divided into subgroups on the basis of being below or above the median pre-pregnancy BMI at index pregnancy, and ΔBMI was compared between groups. Furthermore, cases were divided into two groups on the basis of being below or above the median pre-pregnancy BMI at index pregnancy, and ΔBMI was compared between the groups in terms of two consecutive glucose tolerance profiles.
Data are expressed as number, incidence (%), mean ± SD, or interquartile range (IQR). Comparisons between groups were made using Welch's t-test, χ2 tests, or Fisher tests. Comparisons among groups were made using the Kruskal-Wallis test. Post-hoc analysis by Steel’stest was performed to determine significant differences. Probability values < 0.05 were considered significant.
Results
The designated the study period yielded 183 cases of 149 women diagnosed with GDM at least once in two consecutive pregnancies. Study cases were divided into three groups according to two consecutive glucose tolerance profiles, and comprised GDM/GDM (n=45), GDM/NGT (n=33), and NGT/GDM (n=105).
As seen in Table 1, age at index pregnancy, parity, and duration of breast feeding until subsequent pregnancy did not differ among the three groups. Pre-pregnancy BMI at index pregnancy and baby birth weight at index pregnancy in the GDM/NGT group were significantly lower than those of the GDM/GDM group (p<0.05 and p<0.05, respectively). The median pre-pregnancy BMI of the 183 cases at index pregnancy was 21.2.
|
Study group |
NGT/GDM |
GDM/NGT |
GDM/GDM |
p |
|
(n) |
105 |
33 |
45 |
|
|
Age (years) |
30.6 ± 4.2 |
32.2 ± 4.3 |
32.4 ± 5.1 |
NS |
|
Parity (>1) |
12 |
0 |
5 |
NS |
|
Pre-pregnancy BMI |
23.0 ± 4.4 |
21.4 ± 3.4 |
24.5 ± 5.5* |
<0.05 |
|
Baby birth weight (g) |
3069.1 ± 600.4** |
2768.5 ± 412.2 |
3002.2 ± 448.6* |
<0.01 |
|
Duration of breastfeeding (months) |
11.3 ± 7.6 |
11.1 ± 6.1 |
10.2 ± 8.1 |
NS |
Table 1: Characteristics of the groups studied at index pregnancy
Results are expressed as number or Mean ± SD.
GDM: gestational diabetes mellitus, NGT: normal glucose tolerance. Comparison among groups was made using χ2 tests and the Kruskal-Wallis test followed by comparison using Steel’s test. NS: not significant, *:p<0.05 vs. GDM/NGT, **:p<0.01 vs. GDM/NGT.
As seen in Table 2, age at subsequent pregnancy did not differ among the three groups. However, significant differences in BMI were observed among the three groups (p<0.01). ΔBMI of the GDM/NGT group (-0.4±1.5) was significantly lower than that of the GDM/GDM (0.5±1.6) and NGT/GDM (1.0±2.1) groups (p<0.01 and p<0.01, respectively).
|
Study group |
NGT/GDM |
GDM/NGT |
GDM/GDM |
p |
|
(n) |
105 |
33 |
45 |
|
|
Age (years) |
33.8 ± 4.3 |
34.9 ± 5.2 |
35.2 ± 4.3 |
NS |
|
Δ BMI |
1.0 ± 2.1* |
-0.4 ± 1.5 |
0.5 ± 1.6* |
<0.01 |
Table 2: Age at subsequent pregnancy and ΔBMI of the study groups
Results are expressed as number or Mean ± SD.
GDM: gestational diabetes mellitus, NGT: normal glucose tolerance. ΔBMI: pre-pregnancy body mass index changes between two consecutive pregnancies. Comparison among groups was made using the Kruskal-Wallis test followed by comparison using Steel’s test. NS: not significant, *:p<0.01 vs. GDM/NGT.
Next, considering the median pre-pregnancy BMI at index pregnancies (21.2), we examined the difference in ΔBMI of each group. In the GDM/NGT group, there was no difference in ΔBMI below (n=21, -0.28±1.13) or above the median pre-pregnancy BMI (n=12, -0.64±2.04, p=0.58, Figure 1). In the GDM/GDM group, there was no difference in ΔBMI below (n=18, 0.51±1.23) or above the median pre-pregnancy BMI (n=27, 0.52±1.82, p=0.97, Figure 1). Furthermore, in cases below the median pre-pregnancy BMI, the GDM/NGT group had lower ΔBMI (n=21, -0.28±1.13) compared with the GDM/GDM group (n=18, 0.51±1.23, p<0.05, Figure 2). In cases above the median pre-pregnancy BMI, there was no difference in ΔBMI between the GDM/NGT (n=12, -0.64±2.04) and GDM/GDM groups (n=27, 0.52±1.82, p=0.11, Figure 2).
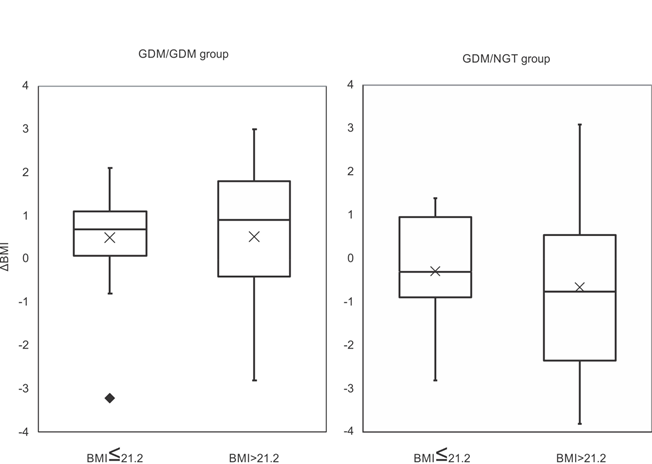
Figure 1: Comparison of ΔBMI differentiated by median pre-pregnancy BMI at index pregnancy in the GDM/NGT and GDM/GDM groups.
The median pre-pregnancy BMI at index pregnancy was 21.2. ΔBMI: pre-pregnancy body mass index changes between two consecutive pregnancies. GDM: gestational diabetes mellitus, NGT: normal glucose tolerance. The interquartile range is represented by a box. The black bar represents the median. The cross represents the mean. The value exceeding 1.5 IQR was represented by closed diamond. Comparisons between groups were made using Welch's t-test.
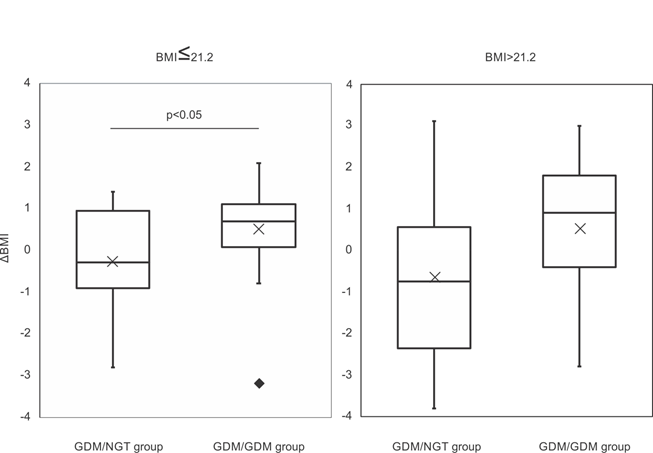
Figure 2: Comparison of ΔBMI differentiated by two consecutive glucose tolerance profiles below and above the median pre-pregnancy BMI at index pregnancy.
The median pre-pregnancy BMI at index pregnancy was 21.2. ΔBMI: pre-pregnancy body mass index changes between two consecutive pregnancies. GDM: gestational diabetes mellitus, NGT: normal glucose tolerance. The interquartile range is represented by a box. The black bar represents the median. The cross represents the mean. The value exceeding 1.5 IQR was represented by closed diamond. Comparisons between groups were made using Welch's t-test.
Discussion
Over half the number of women having GDM develop type 2 DM within the first decade after pregnancy [4]. Therefore, efforts to reduce weight gain after childbirth by engaging in a healthy diet, physical activity, and breastfeeding may prevent or delay the progression of GDM to type 2 DM in women with a history of GDM. It has been reported that suppressing weight gain between two consecutive pregnancies in obese women could prevent the recurrence of GDM [7]. However, since Japanese people are characteristically lean and possess low insulin secretion ability [2, 3, 10], it remains to be determined how controlling the body weight of such a population can prevent the recurrence of GDM. We showed in this study that subtle changes in ΔBMI are associated with GDM recurrence in the Japanese population. In GDM/GDM group, ΔBMI increases regardless of being below or above the median pre-pregnancy BMI at index pregnancy. On the other hand, in GDM/NGT group, ΔBMI decreases regardless of being below or above the median pre-pregnancy BMI at index pregnancy. Additionally, reduced ΔBMI suppresses GDM recurrence in lean Japanese women. However, it seems that the duration of breastfeeding does not affect GDM recurrence in two consecutive pregnancies.
In our study, ΔBMI of the GDM/NGT group (-0.41±1.50) was significantly lower than that of the GDM/GDM group (0.52±1.59). The difference in mean ΔBMI affecting GDM recurrence between groups was about 1 unit and the changes in ΔBMI that affected GDM recurrence were very small in the Japanese population. One study showed that larger changes in BMI between pregnancies affected perinatal prognosis, and in particular women with a BMI gain of 3 or more units had a two-fold higher risk of developing GDM compared with women whose BMI changed from -1.0 to +0.9 units [12]. One meta-analysis also showed that women with a BMI gain of 3 or more units had a two-fold higher risk of developing GDM compared with women who remained in same BMI category or whose BMI changed from −2 to +2 units [13]. Thus, our findings have shown that even subtle changes in BMI within the baseline range for comparison with overseas countries can affect GDM recurrence in Japanese women. Furthermore, the effect of reducing ΔBMI on the recurrence of GDM was significant in leaner Japanese women according to our study. This finding also differed from that observed in an overseas population, where suppressing weight gain between pregnancies was more effective in preventing GDM recurrence in obese women [7]. Thus, the characteristics of GDM recurrence in Japanese women differ from those pertaining to certain overseas populations, and is probably due to the low insulin secretion ability of the Japanese population.
We reported that the proportion of lean GDM women with insulin deficiency and low insulin resistance was relatively high (40%) [3]. Several reports have indicated that impaired insulin secretion is noted during the puerperium [14, 15]. Furthermore, Japanese people can possess higher insulin deficiency at the stage of normal glucose tolerance compared with Caucasians [10]. Therefore, impaired insulin secretion is important for the development of GDM and type 2 DM in Japanese people. Given the predisposition to leanness and impaired insulin secretion in Japanese people, even a slight increase in insulin resistance with a slight weight gain can lead to recurrent GDM. Our findings indicate that the subtle changes in BMI involved in the recurrence of GDM reflect such a scenario. In contrast, when Caucasians become diabetic, the increase in insulin resistance is more pronounced than the impaired insulin secretion ability [2]. Therefore, suppressing weight gain after childbirth may reduce insulin resistance, which in turn may reduce recurrent GDM and the progression of GDM to type 2 DM. One report which showed that suppressing interpregnancy weight gain in obese women reduced GDM recurrence also supports the above scenario [7].
Efforts to reduce weight gain after childbirth by engaging in a healthy diet, physical activity, and breastfeeding may prevent or delay the progression of GDM to type 2 DM in women with a history of GDM. Several reports have shown the effectiveness of breastfeeding in suppressing the onset of type 2 DM [16, 17]. According to one meta-analysis, women who engaged in longer periods of breastfeeding demonstrated more promising characteristics such as significantly lower BMI, higher insulin sensitivity index, and fasting glucose. Additionally, the positive effect of breastfeeding was more prominent in the extended follow-up period [17]. On the other hand, the effects of breastfeeding on recurrent GDM are not always clear [18]. In our study, the duration of breastfeeding did not appear to affect GDM recurrence in two consecutive pregnancies. The short period between pregnancies in our cases may be one reason that our study did not find an association between breastfeeding and the recurrence of GDM. The positive effects of breastfeeding are diverse and extend beyond weight loss [18]. Further studies are needed to delineate the relationship between breastfeeding and the recurrence of GDM.
There were several limitations in our study. Firstly, the number of cases registered in our study is relatively small and there were multiple cases for the same woman. It is necessary to conduct the study with a larger number of cases and where there is no overlap. Secondly, there was a lack of information regarding factors other than breastfeeding related to interpregnancy weight change. It is necessary to evaluate several factors associated with interpregnancy weight change, such as healthy eating, physical activity and intensity of breastfeeding, which may provide effective means of weight control.
In conclusion, subtle changes in ΔBMI are associated with GDM recurrence in Japanese women. In order to prevent or delay the progression of GDM to type 2 DM, information based on the endocrinological characteristics of the Japanese population is crucial. We found that reduced ΔBMI suppresses GDM recurrence in lean Japanese women. Even if women are lean, efforts to reduce subtle weight gain after childbirth would prevent future type 2 DM progression.
Funding sources
This study was supported by a Grant-in-Aid for Clinical Research from the Miyazaki University Hospital.
Disclosure statement
The authors declare no conflict of interest.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 1 (2013): S67-S74.
- Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 66 (2004): S37-S43.
- Furukawa S, Kobayashi Y. Leaner Women with Impaired Insulin Secretion Accounts for about 40% of Gestational Diabetes Mellitus in Japan. J Pregnancy 2019: 7578403.
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25 (2002): 1862-1868.
- Schwartz N, Nachum Z, Green MS. Risk factors of gestational diabetes mellitus recurrence: a meta-analysis. Endocrine 53 (2016): 662-671.
- Nohira T, Kim S, Nakai H, Okabe K, Nohira T, Yoneyama K. Recurrence of gestational diabetes mellitus: rates and risk factors from initial GDM and one abnormal GTT value. Diabetes Res Clin Pract 71 (2006): 75-81.
- Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol 117 (2011): 1323-1330.
- Huopio H, Hakkarainen H, Pääkkönen M, Kuulasmaa T, Voutilainen R, Heinonen S, Cederberg H. Long-term changes in glucose metabolism after gestational diabetes: a double cohort study. BMC Pregnancy Childbirth 30 (2014): 296.
- Yoshiike N, Miyoshi M. Epidemiological aspects of overweight and obesity in Japan--international comparisons. Nihon Rinsho 71 (2013): 207-216.
- Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 36 (2013): 1789-1796.
- Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 19 (2010): 212-228.
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 368 (2006): 1164-1170.
- Oteng-Ntim E, Mononen S, Sawicki O, Seed PT, Bick D, Poston L. Interpregnancy weight change and adverse pregnancy outcomes: a systematic review and meta-analysis. BMJ Open 8 (2018): e018778.
- Katayama H, Tachibana D, Hamuro A, Misugi T, Motoyama K, Morioka T, et al. Sustained Decrease of Early-Phase Insulin Secretion in Japanese Women with Gestational Diabetes Mellitus Who Developed Impaired Glucose Tolerance and Impaired Fasting Glucose Postpartum. Jpn Clin Med 6 (2015): 35-39.
- Kugishima Y, Yasuhi I, Yamashita H, Fukuda M, Kuzume A, Sugimi S, et al. Risk factors associated with abnormal glucose tolerance in the early postpartum period among Japanese women with gestational diabetes. Int J Gynaecol Obstet 129 (2015): 42-45.
- Horta BL, de Lima NP. Breastfeeding and Type 2 Diabetes: Systematic Review and Meta-Analysis. Curr Diab Rep 14 (2019): 1.
- Ma S, Hu S, Liang H, Xiao Y, Tan H. Metabolic effects of breastfeed in women with prior gestational diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab Res Rev 35 (2019): e3108.
- Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care 30 (2007): 1314-1319.19. Del Ciampo LA, Del Ciampo IRL. Breastfeeding and the Benefits of Lactation for Women's Health. Rev Bras Ginecol Obstet 40 (2018): 354-359.
- Del Ciampo LA, Del Ciampo IRL. Breastfeeding and the Benefits of Lactation for Women's Health. Rev Bras Ginecol Obstet 40 (2018): 354-359.


 Impact Factor: * 1.1
Impact Factor: * 1.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 