Transient Threshold Abundance of Haematobia irritans (Linnaeus, 1758) In Cattle Under Integrated Farming Systems
Article Information
Ana Isabella Iura Schafascheka1, Thales Baggio Portugalb1, Alexandre Filusb, Anibal de Moraesb, André de Camargo Guaraldoc#, Izanara Cristine Pritscha, Marcelo Beltrão Molentoa*
a Laboratory of Veterinary Clinical Parasitology, Department of Veterinary Medicine. Federal University of Parana. Curitiba, Paraná, Brazil.
b Plant Science Laboratory, Department of Agronomy. Federal University of Parana. Curitiba, Paraná, Brazil.
c Ecology and Behavior Laboratory, Department of Biology. Federal University of Parana. Curitiba, Paraná, Brazil.
1 Both authors contributed equally to this work.
# Present address: Department of Zoology. Federal University of Juiz de Fora. Juiz de Fora, Minas Gerais, Brazil
*Corresponding Author: MB Molento R, dos Funcionarios, 1540. Curitiba, PR, Brazil
Received: 24 May 2021; Accepted: 4 June 2021; Published: 18 June 2021
Citation:
Ana Isabella Iura Schafaschek, Thales Baggio Portugal, Alexandre Filus, Anibal de Moraes, André de Camargo Guaraldo, Izanara Cristine Pritsch, Marcelo Beltrão Molento. Transient Threshold Abundance of Haematobia Irritans (Linnaeus, 1758) In Cattle Under Integrated Farming Systems. International Journal of Plant, Animal and Environmental Sciences 11 (2021): 322-340.
View / Download Pdf Share at FacebookAbstract
Haematobia irritans is a hematophagous insect that affects the welfare of cattle reducing weight gain and it is present in most countries. The objective of this work was to determine the prevalence of flies, mainly H. irritans in 36 Red Angus calves under four farming ecosystems in Brazil, and to assess an individual threshold limit to treat for horn fly and to attain better animal welfare and farm sustainability. The animals (n = 9) were allocated in livestock (L), crop-livestock (CL), livestock-forestry (LF) and the full integration of crop-livestock-forestry (CLF) conditions. Adult flies were determined between systems on each animal at weekly intervals and in each environment by counting larvae and pupal stages that emerged from selected dung pads. Individual animals were treated for H. irritans infestation after reaching the transient threshold abundance (TTA) of > 100 flies. The data from 1008 evaluations showed that animals in the CLF had significantly (P < 0.05) more horn flies, than animals from the other systems. The level of infestation by horn flies was strongly influenced by the particular ecosystem (P < 0.002), and by the month of the year (P < 0.001). However, the difference in fly numbers did not influence the weight gain of the animals. We found four other genera of the Diptera order (Brontaea spp., Cyrtoneuropsis spp., Fannia spp. and Morellia spp.) that emerged from dung pads. The data suggests that it may be possible to perform evaluations in one animal at a time to control H. irritans with no impact to the performance of the animals when using a low TTA index of infestation. CLF was considered to be the richest environment, favoring fly populations. In our conditions, cattle and different fly species/genera may coexist using sustainable TTA management protocols at farm level in opposite to the concept of fly eradication or mass acaricidal treatment.
Article Details
1. Introduction
The diptera Haematobia irritans (Linnaeus, 1758) (horn fly) is a hematophagous insect that feeds heavily in cattle blood. The saliva of these insects affect coagulation to counter platelet aggregation by using a restricted antihemostatic factor [1]. Although present in brief peaks throughout the year, the economic impact of horn flies to livestock is enormous, mainly due to their repeated and persistent bites [2]. This continuous aggression causes stress, defensive behavior (i.e., increase cortisol concentration) and reduced welfare conditions, reflecting in the decline of weight gain and milk yield [3, 4]. For this reason, horn fly infestation is associated with relevant economic losses in beef cattle worldwide, particularly when fly counts are above 200 flies/animal [5]. It is estimated that in Brazil, the economic impact related to horn fly is approximately US$ 2.6 billion/year [6].
Horn flies have a varying preference for cattle of different colors, breed, and category, as the documented preference is also for adult male animals [7]. This factor is probably due to the lower mobility and sensitivity of bulls, associated with the higher concentration of testosterone [8]. There is also a higher preference by flies for dark-haired animals when compared to lighter-haired ones, particularly when there is a heavy infestation [9]. Scasta and Smith [10], observing the infestation of flies in black and white cows, reported that dark-haired cows were more attacked by flies. The authors concluded that this was due to differences in the external temperature of the animals affecting the insect thermoregulation. There are also differences in the infestation of flies depending on the species of the animal in question. In this sense, Bos indicus are more susceptible to H. irritans than B. taurus, although this difference might not be so evident as for the cattle-tick Rhipicephalus microplus infestation [11]. Barros [12], reported that Nellore cows can be classified as either fly susceptible or resistant, according to their level of infestation, but the author did not stablish a threshold number, determining by above (twice the count) or below (half the count), the mean fly numbers on the herd. Moreover, the authors did not measure any performance parameters between individual animals in Pantanal, Brazil.
Other dipterans may be sympatric with H. irritans, such as Brontaea spp., Cyrtoneuropsis spp., Fannia spp. and Morelia spp. Some of these genera have an enormous diversity in Brazil and are involved in carcass decomposition [13, 14]. The Brontaea spp. genus can be attracted to animal feces by local fly species and may be responsible for differences in the population dynamics of the other flies [15] by altering the fecal pad and preying upon Haematobia sp., for example [16]. The Cyrtoneuropsis spp. genus has more than 35 species, and is very numerous in the state of Parana, as well as other regions of Brazil [17]. Some Cyrtoneuropsis species have been used as ecological indicators of forest disturbance. In relation to Fannia spp., their eggs and larvae may be found in decomposing organic substances and feces, but although there are several species of Fannia spp. in urbanized and rural areas [18] only a few species have veterinary or medical importance. This fly may be a carrier of the larvae of the botfly Dermatobia hominis [19].
Feces from livestock plays an important role in Diptera development at local ecological areas, from egg deposition to adult immersion from pupae, and their spread [20]. The authors suggest that cattle feces may be associated with fitness cost for fecundity, longevity, and fly survival, being a less suitable environment, when compared to dog feces. Therefore, if only cattle feces are available, some flies (i.e., Musca domestica) must travel quite considerably to have a successful life cycle.
The main beef cattle farming system used in Brazil and in tropical and subtropical areas of South America is extensive with suppressive treatment regimens to control horn fly - with increasing drug resistance reports [21]. Extensive farming is usually associated with low productivity and low pasture quality [22]. In contrast, integrated ecosystems have been used to diversify and associate different agroecological systems, providing greater efficiency in the use of local inputs (i.e. agriculture, forestry, and animal protein), enriching the diversity of the environments and making the most of the area's potential. The integration of crop-livestock-forestry systems (CLF) is the most complex farming alternative, associating agriculture, livestock, and forestry activities in the same area to produce grains, milk, meat, wood, and their by-products throughout the year [23]. The objective of the present study was to determine and compare the prevalence of flies, mainly H. irritans, in cattle raised under integrated farming conditions in Brazil, applying a newly developed transient threshold abundance (TTA) protocol of flies. We looked into weight gain, production system, climate, and season (month), as possible risk factors for fly prevalence and impact.
2. Materials and Methods
2.1 Study area
The study was conducted at the Center of Technological Innovation in Agriculture, NITA of the Federal University of Parana, UFPR, in the city of Pinhais, (25o 24’ 4.31’’ S, 49o 7’ 15.02’’ W), Southern Brazil. The experimental agrosystem area was stablished in 2010. The local climate is subtropical humid (Cfb) according to the Köppen-Geiger climate classification [24] with an average annual rainfall of 1.400 mm and an average temperature of 17oC (range of 12.5 to 22.5ºC). Winters are subjected to few but severe frost.
2.2 Experimental farming systems at NITA
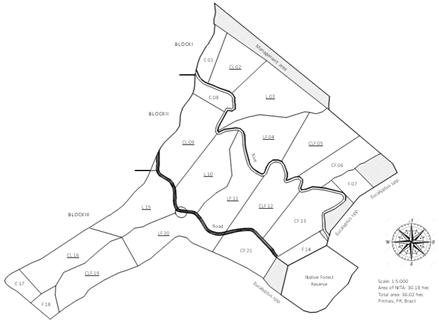
The experimental area of 20.87 ha was divided into 12 paddocks, ranging from 1.2 to 2.2 ha and cultivated with Megathyrsus maximus cv. Aries forage. The experimental design was a randomized block with four agroecosystems: livestock (L), crop-livestock (CL), livestock-forestry (LF), and CLF. Each of the four treatments were distributed in the area with three replicates: Block I, II, and III (Figure 1). Although corn (Zea mays) was used as the main crop production in the areas, it was planted once every three-years in the CL and CLF. It is important to add that CL and CLF areas were not used for crop production during the present work. In 2013, Eucalyptus bentamii trees were planted in the LF and CLF treatments. The percentage of shaded areas in the present protocol was 37% in the LF and 34.3% in the CLF, having no influence to adjacent treatments (i.e., pasture quality).
2.3 Animal groups
Thirty-six Red Angus cross castrated male calves were used for this experiment. The animals stayed for 10 months at NITA, from June 2017 to March 2018. At the beginning of the trial, the animals were 11 months old with an average live weight (LW) of 230 kg (± 27.64 kg). Nine animals, three in each block (i.e., L1, L2, and L3) were included in the four treatments and were maintained on pasture, subjected to their ecological system the entire time of the evaluations.
2.4 Evaluation of immature life stages and adults in fecal pads
The number of larvae and pupae stages and adult flies was determined in each system from November 2017 to the beginning of February 2018, representing the last period of the fly season, and from late February to March 2018, right after the long rains. The methodology consisted in identifying by convenience three fresh fecal pads on pasture in each treatment, every two weeks. The samples were then protected with metal crates (30 x 30 x 30 cm) in the morning to prevent destruction by the animals. Four to six days later, the fecal material was collected to obtain the free-living immature stages (larvae and pupae), according to Oliveira et al. [25]. The entire fecal pad, including some soil underneath the fecal mass was homogenized in a container in 1.5 L of water. Slowly, the mixture was cleaned by filtering at 20, 5.0, and 1.0-mm sieves, looking for larvae and pupae with an approximate size of 6.0 x 3.0 mm, and 4.0 x 2.5 mm, respectively. The pupae were then placed in 15-cm Petri dishes with 5 g of vermiculite, to create a proper environment, covered with a cotton screen and left at room temperature with approximately 80% humidity. The larvae were placed in a 500 ml glass container with 200 g of vermiculite and fresh and clean bovine feces and covered with a cotton screen. The containers and Petri dishes were left at room temperature (approx. 24oC) and 80% humidity waiting for up to 10 days for the emergence of adult flies. The genus identification of the adult flies was based on their morphological characteristic using taxonomic keys [26] (Table 1). A sampling in April 2018 was also taken to determine the residual fly population in the areas as the animals had left the systems by the end of March.
2.5 Evaluation of horn fly infestation and cattle performance
Horn flies were identified by visual characteristic at approximately 2 m from the animals looking at the head, neck, dorsal line, ribs, belly, and legs. The counting was performed on the animals at weekly intervals for seven months (Sep 2017 to Mar 2018), covering the main horn fly season [12]. The fly counts were always made by the same trained personnel, between 11:00 and 13:00 a.m. based on the protocol suggested by [27] with modifications. The local average temperature was between 19.0 and 22.5oC with a maximum variation during the evaluation period from 8 to 33oC, according to the SIMEPAR weather station.
For the fly evaluation, we have established a transient threshold abundance (TTA) index of fly count, represented by, 0 (zero): no flies, 1: representing approximately 1 to 10 flies in one side of the animal, 2: 11 to 25 flies, 3: 26 to 50 flies, and 4: >51 flies (> 100 flies on the animal). The TTA parameters were selected as we did not want the animals to have any welfare implications, establishing a low upper threshold for the treatment. When the number of flies reached level 4, the animals were individually treated with fluazuron at 2.5 mg/kg in combination with fipronil at 1.25 mg/kg (Tick Gard, MSD Saúde Animal, Brazil) pour-on. The treated animals stayed isolated for 6 h before being integrated with their group to avoid licking and rubbing behaviors and the passive drug contamination to untreated animals or to the environment.
Some animals were never treated, showing no or low fly numbers during the entire period. In this case, untreated animals were considered as the control group when comparing their weight gain to the treated ones. Phenotypic tolerant individuals are preferable to form the control group instead of continuously suppressing (treating) a designated group of animals, which do not express natural resilience, benefiting only from the chemical advantage [11]. This decision was taken, and we relied on the natural resilient response of the animals against the parasitic challenges, considering the degree of infestation, the systems they were in and their individual performance.
The animals were weighted at every 28 days, being submitted to a 12 h fasting period before the procedure. The daily weight gain (DWG) was calculated using the animals’ weight values, subtracting the posterior weighting of the previous date, dividing by the number of days between weightings. Dermatobia hominis and R. microplus were also counted on the animals and TTA treatment with the same drug was given according to [13, 28].
2.6 Climatic conditions
Temperature and rainfall data were collected from the Agrometeorological Monitoring System - Agritempo of the Meteorological System of Parana - SIMEPAR, during the entire period of the study. As weather conditions during winter were very harsh with no flies, the presence of flies started from September 2017 on.
2.7 Statistical analyses
We ran two sets of models. The first model evaluated the influence of the production system in cattle infestation by fly counts, using a cumulative link mixed model, CLMM. This is an extension of the generalized linear models, GLM for an ordinal variable response (Agresti, 2002), i.e. the degree of infestation by horn flies (scale from 0 to 4). Due to differences in pasture quality, the four production systems (L, LF, CL, and CLF) and month, were used as predictors for the model in which we controlled for likely biases due to multiple samplings per individual, considering the animal `id` as a random term effect. We have assessed the significance of each predictor variable by a likelihood ratio (LR) between the full and simplified models and the significance of each predictor level through their predicted marginal means (least-square means) using the Bonferroni correction of the significance level.
The second model evaluated the influence of fly count on cattle DWG through a linear mixed model LMM, considering the infestation degree and month as predictors for weight gain. This model was preferred over a linear model LM, based on the low between-individual variance (σ² ± SD = 0.02 ±0.13), and the best fit of LM over the LMM, as measured by the Akaike Information Criteria (ΔAIC = 67.70; c² = 123.8; P < 0.001; Table S1). We ran post-hoc Tukey pairwise comparisons followed by the Bonferroni correction of the significance level, testing the significance of each predictor factor. The influence of the treatment for ticks and other parasites was also taken into the analysis. All analyses were run using packages ordinal [29] and multcomp [30] in R v. 3.4.2 [31], assuming α = 0.05.
The results from the EMM, Akaike criteria, and LMM variations are shown in the Supplementary material (Table S1 to S5).
3. Results
3.1 Evaluation of immature life stages and adults in fecal pads
We found four genera of the Diptera order, Brontaea spp., Cyrtoneuropsis spp., Fannia spp. and Morellia spp. The number of recovered adult flies from all four systems is presented in Table 1. There was a large variation (P < 0.05) on the number of flies in each ecosystem and in between samplings being higher in February 2018. We could not determine the dependent factor for the observations, but the overall number of flies decreased as the volume of rainfall increased. Temperature had no major influence in fly prevalence, as it stayed in the range of 16 to 22oC. Cyrtoneuropsis was the genus that showed the highest prevalence in the CL in February (91.5%), and in CLF in March (74%). In April, 74% of the collected flies were Brontaea spp. in the CLF. The presence of this fly was constant in all systems. We have counted Fannia spp. Morellia spp. in very few numbers in L and LF. No larval or pupal stages of H. irritans were not recovered from the collected dung pads.
3.2 Evaluation of horn fly infestation on cattle
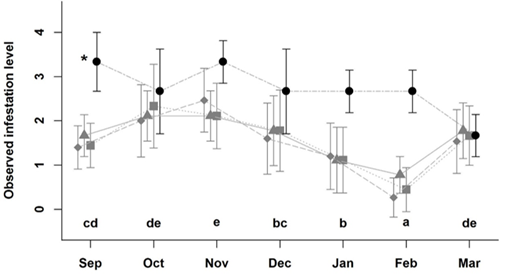
During the study, animals were evaluated 1.008 times to determine individual fly counts. The likelihood ratio analysis (Table S1 & S2) showed that the degree of infestation by horn flies was strongly influenced by the production system (full versus simplified model: LR = 14.5, gl = 3, P = 0.002), and by the month of the year (LR = 297.5, gl = 6, P < 0.001). Animals in CLF had significantly higher fly counts than animals from the other systems throughout the entire observation period (September 2017 to March 2018) (CLF: -1.47; CL: -2.66; L: -2.89; and LF: -2.87; Table S3) (P < 0.05). Overall, significantly lower odds of horn fly infestation were observed in January and February 2018 and significantly higher odds during October, November, and December 2017 (Figure 2). No flies were observed on the animals during the winter months of July and August 2017.
3.3 Evaluation of cattle weight gain
The monthly average number of horn flies on each animal per agrosystem was not considered high during the entire period, as shown in Table 2. In the CL system, two selective treatments were performed, both to the same animal. In the CLF, five treatments were necessary, being three in the same animal. In the LF, two treatments were used to the same animal and in the L system no treatment was necessary. All treatments were performed in 2017, describing the peak of the fly infestation in all areas but higher in the CLF areas (Figure 2). We did not observe any licking behavior between animals after treatment.
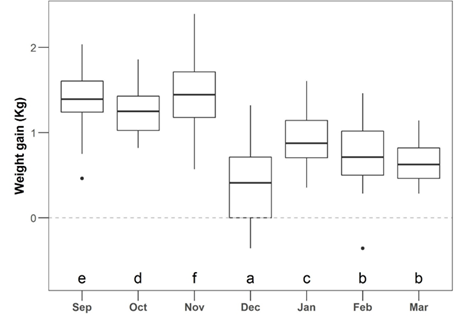
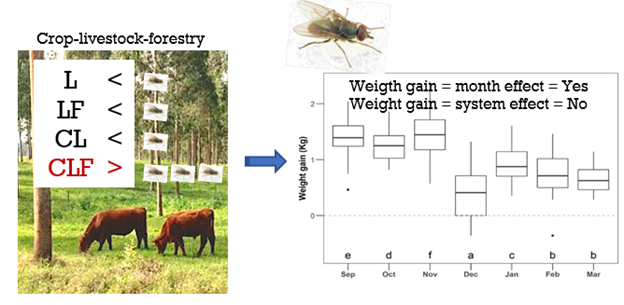
The variation in the individual DWG was considered independent of the infestation level (LR = 5.9; P = 0.21; Table S4) but suffered a significant effect through different months (LR = 1203.5; P < 0.001; Figure 3; Table S4).
3.3 Evaluation of horn fly and other fly prevalence vs. climatic conditions
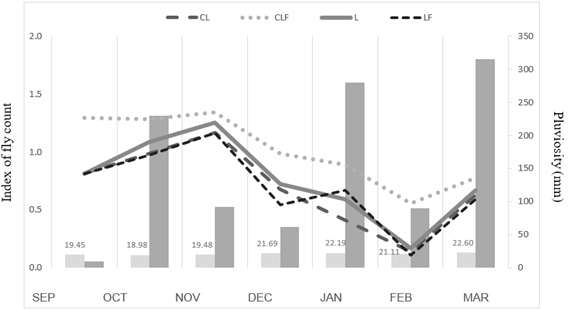
The correlation between the presence of horn fly and climatic conditions (temperature and rainfall), from September 2017 to March 2018 are shown in Figure 4. Temperature had a maximum variation from 8 to 33°C, while precipitation ranged from 10 (Sep/2017) to 315 mm/month (Mar/2018). The concentration of horn flies based on the TTA 0 to 4 index and temperature showed a strong and negative correlation (-0.781), indicating that the higher the temperature, the lower the presence of horn flies. In relation to precipitation, a low and negative correlation (R2 = -0.291) was found.
Figure 1: Experimental area of the Nucleus of Innovation Technology in Agriculture, NITA, in Pinhais, PR, Brazil. The area is divided in three blocks, each containing seven treatments: L: livestock, C: Crop, and F: forestry and their combination. Underline indicates the treatments that were used in each block.
Figure 2: Observed index of infestation (+/- standard deviation) of Haematobia irritans level of infestation on cattle in crop-livestock-forest (black circles), crop-livestock (grey squares), livestock-forestry (grey triangles), and livestock (grey diamonds), from September of 2017 to March 2018 in Pinhais, Brazil.
Legend: Equal letters identify months with an overall similar value of infestation irrespective of the production the system. *: Cattle in the CLF system had significantly higher infestation levels in comparison to those in the other systems.
Equal letters identify the months with similar levels of weight gain (kg) according to Bonferroni correction (α = 0.05) was minimized by the addition of a small noise (i.e., jitter). Dots: the two dots in the figure represent two outline animals. Sep = September, Oct = October, Nov = November, Dec = December, Jan = January, Feb = February, and Mar = March.
Figure 4: Average temperature (oC) (light-grey bars) and pluviosity (mm) (grey bars), overlapped by index of fly count (refer to section 2.5), in the different systems: livestock (L), crop-livestock (CL), crop-livestock-forestry (CLF) and livestock-forestry (LF) system from September 2017 to March 2018, in Pinhais, Brazil.
4. Discussion
4.1 Evaluation of immature life stages and adults in fecal pads
According to the genus of the flies, we found Brontaea spp. as the most prevalent larva in bovine fecal pads, during all months and in all livestock ecosystems. Cyrtoneuropsis spp. was determined in very high numbers in all systems in February and in CLF in March, demonstrating a peak of its presence late in the summer, as also reported by [32], almost disappearing in April 2018 (residual sampling). Fannia spp. and Morelia spp. had both very low counts with no preference for any ecosystem interaction due to its large range of environmental conditions.
Brontaea spp. may have influenced the population dynamic of the other flies by altering fecal pads, which could have been an important factor for the absence of H. irritans larvae in the areas. Cyrtoneuropsis spp. are useful ecological indicators and were found in high numbers in the beginning of the samplings, decreasing considerably by the end of the summer. Fannia spp. have little importance to animals [18] but it can be the carrier of eggs of D. hominis [19]. We are particularly interested to study the impact of Fannia spp. in the population dynamics of the botfly and also to determine their ecological correlation with season, and animal welfare.
In the present study, the emergence of H. irritans stages (larval, pupal or adults) was not observed from the fecal pads. This result may be associated with the low presence of horn flies on the animals, the local mild climate conditions, the exclusive cattle manure, a long pupal diapause, and the number of fecal samples that were used for fly identification. [33], evaluated the emergence of H. irritans from fecal pads of Nellore and Pantaneiro cattle in Mato Grosso, Brazil. The authors reported that from the 112 pupae that were collected, adult emergence occurred in approximately 60%. Another condition that may be responsible for the low horn fly emergence in the integration environment is the competition factor, as several other bacteria and insects could be responsible for parasitizing the free-living stages of H. irritans, causing the interruption of the biological life cycle in the feces and soil, maintaining the parasite in low numbers, as previously observed in the same study area [34]. Similar results were also reported by [35] in a study associating the prevalence of arthropods in bovine dung pats. In that case, the Muscidae family was the most abundant among the Diptera, constituting 61.4% of all insects. However, similarly to our study, no horn flies were collected, as a significant number of flies from the Staphylinidae family were responsible for most of the predation of the horn fly pupae in feces [35].
As many factors could play a role in affecting the free-living stages of all diptera in the environment, we still have to evaluate the presence of horn flies at NITA’s multifaceted ecological systems. Lastly [20], found that cattle manure may be associated with the reduction of fecundity and survival of M. domestica, being a less suitable environment. In our integrated treatments, there were other animals, including poultry and swine, being raised in a range of a few kilometers. Therefore, we still need to determine if H. irritans could have laid their eggs in neighboring areas to complete their life cycle.
As flies may provide an intrinsic ecological interaction, contributing as a major player in ecosystem services, we have just provided more understandings towards another integration that shall reduce the dependence of artificial/external control. We should, therefore, determine how these insects may influence local dynamics that could result in major impact to control other species, reflecting in a safe condition to maintain livestock in a sustainable equilibrium with a significant reduction of the use of acaricidal products.
4.2 Evaluation of horn fly infestation on cattle
The average TTA index of infestations during the 2017/2018 season were 0.661, 0.663, 0.756, and 0.940 in the LF, CL, L, and CLF ecosystems, respectively. An aspect to be considered is the fact that during the study four target selective treatments were performed to control R. microplus using the same acaricide, mainly in February and March. Although cattle ticks were observed 30 days after the animals entered the area, the number of treatments due to low fly numbers did not affect the DWG of the animals with no impact in the number or the distribution of flies in the different systems. Therefore, it is believed that, even though this may have been a factor to consider, the fly season did not impose a major challenge to the animals with a small number of treatments (n=9) directed to control H. irritans. As mentioned before, all treated animals stayed in separate paddocks after treatment to avoid the licking and rubbing behavior. Ivermectin is an injectable acaricide drug that may be transfer by indirect self- or allo-grooming in cattle [36], resulting in partial efficacy to untreated animals [36]. We still have to determine this sub-dose effect of acaricides against H. irritans, as well as the long-term effects of ecosystem services when using fewer chemical doses.
The CLF area presented a significantly higher concentration of horn flies on the animals in relation to the other systems. The greater environmental complexity found in CLF may have imposed indirect advantages to fly continuance, i.e., the more restful the animals, the more time the flies could stay on them. [25] verified a different number of horn flies on animals in a LF system in comparison to cattle maintained in conventional L areas. The authors associated the lower number of horn flies to the more complex and higher biodiversity environment of silvopastoral areas. According to the same authors, these environments have a greater proliferation of different arthropods that are responsible for creating an environment of greater competitiveness, resulting in a lower emergence of H. irritans. This hypothesis was somewhat supported by our data, evidenced by the prevalence of Brontaea spp., Cyrtoneuropsis spp., Fannia spp. and Morellia spp. flies that emerged from the L and LF areas, although more pupae of these flies were found in the area associated with the triple integration. As pointed out by [37], the CLF system has a much more complex environment, which could have a higher ecological influence than single systems. We consider that other interactions (cattle resting time, shadow) may have also influenced the high prevalence of horn fly populations. One major difference that is worth to mention, is that the above referenced studies did not monitor other species of flies, centering only on horn fly.
The modification of pasture structure in the CLF, LF and LC environments, due to the combination between crop and forest components, may favor the maintenance of the insects. This dynamic of different vegetation structure and the increase in parasite numbers was observed by Scasta et al. [38]. In LF and CLF full interaction, pasture areas were used in association with forest to promote better temperature control and to reduce solar radiation and wind speed [25], a condition that could favor the protection of H. irritans and other flies in the ecological areas. The lower solar/radiation incidence allows the feces to remain moist and conducive to the development of different fly larvae. The trees may also exert an effect of reducing the impact of the rain to the soil in the LF and CLF systems. Thus, in situations of excessive rainfall, trees can significantly prevent the destruction of the dung pats, allowing the continuity of the flies’ life cycle.
4.3 Evaluation of cattle weight gain
We did not observe any influence of the TTA of H. irritans on the weight gain of the animals. This fact was probably due to the low average number of horn flies, even when high fly counts (> 100/animal) were determined. Similarly, constant low infestations were also reported in the literature with high fly variation in between seasons [12]. [39] showed that untreated calves had an average infestation of 109 flies/animal, which caused a significant loss of 20 kg/animal in weight gain, when compared to animals that received one diazinon ear tag or a triple drug combination treatment, over a period of 150 days. It must be explained that animals from the above study were not individually evaluated, with animals receiving a blanked treatment based on an average fly number. Therefore, the average weight loss shall not be attributed to ‘20 kg/animal’ as weight was recorded as a total loss, diluting the individual animal effect. This interpretation misleads the fly impact focusing only in the short-acting chemical benefit. [39] reported that infested cattle would manifest clinical signs of H. irritans such as irritability, using the head to avoid flies and forming groups with other animals to defend themselves. The above data have a clear impact on animal welfare [4, 40], and should not be allowed in any farm or experiment, as these conditions undoubtedly have a negative impact as some animals can have more than 800 flies, directly impacting in poor performance and suffering. [41], evaluated beef replacement heifers, demonstrating an increase in total weight gain of 14% of treated animals, compared to the untreated control group. [42], found similar results with a significantly higher weight gain of treated cattle, in comparison to untreated ones. Again, the two previous studies did not classify the number of flies per animal to decide their most appropriate time for treatment, missing the opportunity to individually correlate weight loss and fly count. Taking all the above data together, we believe that there should be a threshold number of flies before deciding for the individual treatment. The TTA number should be fixed, even though one may deal with different cattle breeds, climate, micro-environment, and farm management, which can inflict in transient conditions. At this time, and differently from the historically 200-fly limit, we are suggesting a predetermined TTA index of 0 to 4 (refer to Material and Methods) to be considered for validation in different geographical areas, to establish the impact of this recommendation on performance, i.e., weight gain, which is one aspect to measure animal welfare, along with irritability, tail wagging, and anemia.
As observed in the present study, there were important advantages when using the individual TTA monitoring strategy. The concept of treating the most infested animals is the basis for significantly reducing the use of ectoparasiticides that may also have a direct reduction in farm cost and environmental contamination, matching the modern ecohealth and environmental safety standards [43]. The use of this strategy is essential when considering cattle selection for resilience, supported by heterosis [44]. In this context, it is important to note that more than 60% of all treatments (susceptible disease trait) were recurrent to the same group of animals. Moreover, the selective regime used in the present study showed a 70% reduction in the fly and tick treatments, when comparing with the data from the previous season at NITA (T. Portugal, personal information). Regarding fly population, untreated animals would also allow a vast numerical advantage of fly survival with beneficial susceptible traits [45, 46]. Not only that, but the concept of refugia still need to be tested in horn fly. For this, the adoption of the TTA strategy is unquestionably desirable in Brazil and other countries with large H. irritans resistant populations.
The expansion of livestock integrated systems, such as CL, LF and CLF focus on sustainable agrosystems and the importance of their long-term environment and ecosystem services [47]. This is consistent with the sustainable agricultural intensification and high yields in land-efficient ecosystems. Even though we still need to look into societal and economic conditions, as well as environmental, industrial and the latest pressing issues of animal diversity and welfare [48]. In this scenario, the TTA strategy for parasite control can play a central role, as one of the most innovative control practices in opposition of mass treatment in fixed calendars [28], which greatly affects biodiversity, increase soil degradation and water toxic levels. Therefore, the adoption of the individual evaluation of cattle to control horn flies shall be supported, helping to mitigate drug resistance, and environmental implications of intensive agricultural use. Furthermore, we must support holistic systems looking for long-term management approaches that would protect local agroecological systems, extending the meaning of sustainable agriculture with high animal welfare and stewardship.
5. Conclusion
The integration of CLF had the highest horn fly count of all livestock agrosystems possibly due to its high complex environment. The presence of horn fly did not affect the performance of the animals, as we had a low-to-mild level of infestation during the period, allowing the animals to express their natural resilience. Month was the most important predictor for horn fly with significant climate and ecological influences. It was evident the occurrence of horn flies repeatedly in 60% of the animals. Our work suggests that it may be possible to perform individual TTA evaluation to control H. irritans with no significant impact to the performance of the animals constantly measuring fly infestation. We consider that the TTA is an efficient tool and may be incorporated in agroecosystems, providing a strong integration with ecosystem services due to the coexistence of cattle and the presence of a diverse number of fly species.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES/Grant number 001, Brazil.
Conflict of interest
The authors declare to have no conflict of interest.
Acknowledgment
The authors would like to thank all the graduate students of the School of Agronomy and Animal Science that participated in the data collection.
References
- Cupp EW, Cupp MS, Ribeiro JM, et al. Blood-feeding strategy of Haematobia irritans (Diptera: Muscidae). J Med Entomol 35 (1998): 591-595.
- Haufe WO. Productivity of the cow-calf unit in range cattle protected from horn flies Haematobia irritans (L.) by pesticidal ear tags. Can J Anim Sci 66 (1986): 575-589.
- Byford RL, Lockwood JA, Sparks TCA. Novel resistance management strategy for horn flies (Diptera: Muscidae). J Econ Entomol 80 (1987): 291-296.
- Vitela-Mendoza I, Cruz-Vazquez C, Solano-Vergara J, et al. Relationship between serum cortisol concentration and defensive behavioral responses of dairy cows exposed to natural infestation by stable fly, Stomoxys calcitrans. J Dairy Sci 99 (2016): 9912–9916.
- Steelman CD, Brown AH, Gbur EE, Tolley G. Interactive response of horn fly (Diptera: Muscidae) and selected breeds of beef cattle. J Econ Entomol 84 (1991): 1275-1282.
- Grisi L, Leite RC, Martins JRS, et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Braz J Vet Parasitol 23 (2014): 150-156.
- Steelman CD, Gbur EE, Tolley G. Individual variation within breeds of beef cattle in resistance to horn fly (Diptera: Muscidae). J Med Entomol 30 (1993): 414- 420.
- Dobson RC, Kurz FW, Sanders DP. Attraction of horn flies to testosterone-treated steers. J Econ Entomol 63 (1970): 323-324.
- Franks RE, Burns EC, England NC. Color preference of the horn fly, Haematobia irritans, on beef cattle. J Econ Entomol 57 (1964): 371-372.
- Scasta JD, Smith T. Commingled black and white cows (Bos taurus; Angus and Charolais) in high-elevation rangeland are differentially parasitized by Haematobia irritans. A Prod Sci (2018).
- Molento MB, Fortes FS, Buzatti A, et al. Partial selective treatment of Rhipicephalus microplus and breed resistance variation in beef cows in Rio Grande do Sul, Brazil. Vet Parasitol 192 (2013): 234-239.
- Barros ATM. Dynamics of Haematobia irritans (Diptera: Muscidae) infestation on nelore cattle in the Pantanal, Brazil. Mem Inst Oswaldo Cruz 96 (2001): 445-450.
- Barbosa RR, Mello-Patiu CA, Mello, RP, et al. New records of calyptrate dipterans (Fanniisae, Muscidae and Sarcophagidae) associated with the decomposition of domestic pigs in Brazil. Mem Inst Oswaldo Cruz 104 (2009): 923-926.
- Skidmore P. The biology of the muscidae of the world. Kluwer Academic Publ. Hingham, MA, USA (1985).
- Carvalho CJB, Pont AC. A revision of new world Brontaea Kowarz (Diptera, Muscidae). Braz J Zool 14 (1997): 723-749.
- Cabrera-Walsh G, Cordo HA. Coprophilous arthropod community from Argentina with species of potential use as biocontrol agents against pest flies. Env Entomol 26 (1997): 191-200.
- Pont AC, Pamplona DM. A note on the genus Paracyrtoneurina Pamplona, 1999 (Diptera, Muscidae). Studia Dipterol 7 (2000): 223-224.
- Marchiori CH. Biology of Fannia pusio (WIEDEMANN, 1830) (Diptera: fanniidae) in the laboratory. Dissertation. Campinas: Campinas State University (1993).
- Wendt LD, Carvalho CJBD. Taxonomy of Fanniidae (Diptera) of southern Brazil – II: New species and key to identification of Fannia Robineau-Desvoidy. Braz J Entomol 53 (2009): 171-206.
- Khan HAA, Shad AS, Akram W. Effect of livestock manures on the fitness of house fly, Musca domestica L. (Diptera: Muscidae). Parasitol Res 111 (2012): 1165-1171.
- Cuore U, Solari MA, Castro-Janer E, et al. Epidemiologia y control de dípteros em estado adulto y larvário em Uruguay. In: Fiel C, Nari A. Ed. Enfermidades parasitarias de importância clínica y productiva em ruminantes. Pp. 571-604, Chap. 25. Montevideo, Uruguay (2013).
- De Carvalho TB, De Zen S. The beef cattle chain in Brazil: evolution and trends. iPecege 3 (2017): 85-99.
- Carvalho PCF, Anghinoni I, De Moraes A, et al. Managing grazing animals to achieve nutrient cycling and soil improvement in no-till integrated systems. Nutr Cycl Agroecos 88 (2010): 259-273.
- Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Sys Sci Disc 11 (2007): 1633-1644.
- Oliveira MCDS, Nicodemo MLF, Gusmão MR, et al. Differential Haematobia irritans infestation levels in beef cattle raised in silvopastoral and conventional pasture systems. Vet Parasitol 246 (2017): 96-99.
- Carvalho CJB. Muscidae (Diptera) of the neotropical region: taxonomy. Dissertation. Curitiba: Universidade Federal do Parana (2002).
- Lima LG, Prado A, Perri SH. Comparison of two methods (visual estimates and filming) for counts of horn flies (Haematobia irritans) (L.) (Diptera: Muscidae). Vet Parasitol 103 (2002): 227-235.
- Molento MB. Parasite control in the age of drug resistance and changing agricultural practices. Vet Parasitol 163 (2009): 229-234.
- Christensen RHB. Ordinal-Regression Models for Ordinal Data. R package version (2015): 6-28.
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biomet J 50 (2008): 346-363.
- R Core Team. R: A language and environment for statistical computing. 3rd Edition. Vienna, Austria, R Foundation for Statistical Computing (2008).
- Moura MO, Carvalho CJB, Monteiro-Filho ELA. A preliminar analysis of insects of medical-legal importance in Curitiba, state of Parana. Mem Inst Oswaldo Cruz 18 (2005): 269-274.
- Sereno FTP, Sereno JRB. Emergence of Haematobia irritans in feces of nelore and pantaneira breed in the pantanal mato-grossense, Brazil. Pesq Agrop Bras 34 (1999): 1705-1709.
- Bischoff AM. Occurrence of Lepidoptera and its parasitoids in Avena strigose and Rumex obtusifolius in crop-forestry system. Dissertation. Curitiba: Universidade Federal do Parana (2018).
- Marchiori CH, De Oliveira AT, Linhares AX. Arthropods Associated with Bovine Dung Pats in Southern Goiás State, Brazil. Neot Entomol 30 (2001): 19-24.
- Laffont CM, Alvinerie M, Bousquet-Melou A, et al. Licking behaviour and environmental contamination arising from pour-on ivermectin for cattle. Int J Parasitol 31 (2001): 1687-1692.
- Luedeling E, Kindt R, Huth N, et al. Agroforestry systems in a changing climate – challenges in projecting future performance. Curr. Opin. Env. Sustain 6 (2014): 1-7.
- Scasta JD, Talley JL, Engle DM, et al. Climate extremes, vegetation change, and de-coupling of interactive fire-grazing processes exacerbate fly parasitism of cattle. Envir Entomol 46 (2017): 191-200.
- Maciel WG, Lopes WDZ, Cruz BC, et al. Effects of Haematobia irritans infestation on weight gain of Nelore calves assessed with different antiparasitic treatment schemes. Prev Vet Med 118 (2014): 182-186.
- Broom DM, and KG Johnson. Stress and Animal Welfare. Chapman and Hall, London, UK (1993).
- De Rouen SM, Foil LD, Mackay AJ, et al. Effect of Horn Fly (Haematobia irritans) control on growth and reproduction of beef heifers. J Econ Entomol 96 (2003): 1612-1616.
- Bianchin I, Koller WW, Alves RGO, et al. Effects of the horn fly, Haematobia irritans (L.) (Diptera: Muscidae) in the weight gain on Nellore cattle. Ciên Rural 34 (2004): 885-890.
- Blair, R. Organic production, and food safety. Wiley-Blackwell Publ., Ames, Iowa, USA (2012).
- Sorensen MK, Norberg E, Pedersen J, et al. Crossbreeding in dairy cattle: a Danish perspective. J Dairy Sci 91 (2008): 4116-4128.
- Chaaban A, Santos VMCS, Martins CEN, et al. Tissue damage and cytotoxic effects of Tagetes minuta essential oil against Lucilia cuprina. Exp Parasitol 198 (2019a): 46-52.
- Chaaban A, Richardi VS, Carrer AR, et al. Insecticide activity of Curcuma longa leaves essential oil and its major compound alfa-phellandrene against Lucilia cuprina larvae (Diptera: Calliphoridae): histological and ultrastructural biomarkers assessment. J Pest Biochem Physiol 153 (2019b): 17-27.
- Bonaudo T, Bendahan AB, Sabatier R, et al. Agroecological principles for the redesign of integrated crop–livestock systems. European Journal of Agronomy 57 (2014): 43–51.
- Balmford A, Amano T, Bartlett H, et al. The environment costs and benefits of high-yield farming. Nature Sust 1 (2018): 477-485.
Supplementary material
A Graphical abstract
Table S1: Likelihood ratio tests showing the importance of both predictors (month and treatment) in explaining the variations of the degree of cattle infestation by horn flies.
|
Model |
DF: Degree of Freedom |
AIC |
LR |
p-value |
|
Full |
- |
3010.2 |
- |
- |
|
Without month |
6 |
3295.7 |
297.5 |
<0.001 |
|
Without treatment |
3 |
3018.7 |
14.5 |
0.002 |
AIC: Akaike Information Criteria; LR: likelihood ratio.
Table S2: Results of the Cumulative Link Mixed Model evaluating the influence of treatment and month on the infestation degree of cattle by horn fly (n=36 individuals).
|
Random effects |
Variance |
SD |
||
|
Individual |
0.2 |
0.45 |
||
|
Fixed effects* |
Estimate (ß) |
SE |
z |
p |
|
February |
-1.9 |
0.25 |
-7.61 |
<0.001 |
|
January |
-0.36 |
0.23 |
-1.54 |
0.12 |
|
March |
1.05 |
0.22 |
4.78 |
<0.001 |
|
November |
1.42 |
0.16 |
9 |
<0.001 |
|
October |
0.99 |
0.16 |
6.22 |
<0.001 |
|
September |
0.6 |
0.22 |
2.71 |
0.006 |
|
CLF |
1.19 |
0.36 |
3.26 |
0.001 |
|
L |
-0.23 |
0.23 |
-1 |
0.32 |
|
LF |
-0.21 |
0.26 |
-0.8 |
0.42 |
*: Reference levels are Crop-livestock treatment and December. CLF: crop-livestock-forestry; L: livestock; LF: livestock-forestry.
Table S3: Pairwise post-hoc comparison testing of the Estimated Marginal Means (EMM) for the differences in cattle infestation by horn fly in four different treatments.
|
Treatment |
EMM |
SE |
Group |
|
L |
-2.89 |
0.18 |
a |
|
CF |
-2.87 |
0.21 |
a |
|
CL |
-2.66 |
0.21 |
a |
|
CLF |
-1.47 |
0.33 |
b |
L: livestock; CL: crop-livestock; LF: livestock-forestry; CLF: crop-livestock-forestry; Group: similar letters indicate similar estimations after a Bonferroni adjustment and considering α=0.05.
Table S4: Relevance of infestation degree by horn flies and month in explaining the daily weight gain of cattle through the comparison of a full model and simplified models without one of the predictors at a time.
|
Predictor |
DF |
AIC |
LR |
p-value |
|
Full |
- |
1122.6 |
- |
- |
|
Without horn fly infestation degree |
4 |
1120.4 |
5.9 |
0.21 |
|
Without month |
6 |
2314.1 |
1203.5 |
<0.001 |
AID: Akaike Information Criteria; LR: Likelihood ratio
Table S5: Results of the Linear Mixed Model evaluating the influence of degree of horn fly infestation (ranging from 0 to 4) and month on cattle daily weight gain (n=36 individuals).
|
Random effects |
Variance |
SD |
|
|
Individual |
0.02 |
0.13 |
|
|
Residual |
0.12 |
0.35 |
|
|
Fixed effects |
Estimate (ß) |
SE |
t |
|
Intercept* |
0.42 |
0.03 |
12.74 |
|
Infestation 1 |
-0.02 |
0.02 |
-0.96 |
|
Infestation 2 |
-0.07 |
0.03 |
-2.36 |
|
Infestation 3 |
-0.02 |
0.06 |
-0.42 |
|
Infestation 4 |
0.02 |
0.11 |
0.13 |
|
September |
0.97 |
0.04 |
24.77 |
|
October |
0.85 |
0.03 |
29.67 |
|
November |
1.1 |
0.03 |
38.7 |
|
January |
0.53 |
0.04 |
13.41 |
|
February |
0.32 |
0.04 |
8.86 |
|
March |
0.26 |
0.04 |
6.45 |
*: Reference levels are Infestation 0 and December.

 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 