Valorization in nectars of pulps from two mangoes varieties (Amelie and Kent) upgraded by exporting companies in Northern Côte d’Ivoire
Article Information
Zoro Armel Fabrice1,3, Touré Abdoulaye1,2*, Miezan Bilé Aka Patrice1,2, Seydou Kouadio Amoulaye1, Soro Yadé Réné3, Coulibaly Adama2
1Laboratoire de Biotechnologie et Valorisation des Agroressources et Substances Naturelles, UFR, Sciences Biologiques, Université Peleforo Gon Coulibaly, BP 1328 Korhogo, Côte d’Ivoire
2Laboratoire de Pharmacodynamie-Biochimie, UFR Biosciences, Université Félix Houphouët-Boigny, 22 BP 582 Abidjan 22, Côte d’Ivoire
3Laboratoire de Biotechnologie, UFR of Biosciences, Université Felix Houphouët-Boigny, 22 BP 582 Abidjan 22, Côte d’Ivoire
*Corresponding Author: Touré Abdoulaye, Laboratoire de Biotechnologie et Valorisation des Agroressources et Substances Naturelles, UFR, Sciences Biologiques, Université Peleforo Gon Coulibaly, BP 1328 Korhogo, Côte d’Ivoire
Received: 09 April 2021; Accepted: 14 April 2021; Published: 23 April 2021
Citation: Zoro Armel Fabrice, Touré Abdoulaye, Miezan Bilé Aka Patrice, Seydou Kouadio Amoulaye, Soro Yadé Réné, Coulibaly Adama. Valorization in nectars of pulps from two mangoes varieties (Amelie and Kent) upgraded by exporting companies in Northern Côte d’Ivoire. Journal of Food Science and Nutrition Research 4 (2021): 066-076.
View / Download Pdf Share at FacebookAbstract
To fight against food insecurity and improve the income of farmers and exporters, valorization in nectars of pulps from unsuitable mango for export remains an alternative way. The present study aimed to produce nectars with pulps of the upgraded mango varieties namely Amelie and Kent from SODIPEX-SARL, an exporting companies of Korhogo (Northern Côte d’Ivoire). Physicochemical and biochemical parameters of the pulps each mango variety were determined before prepared with them various formulations of nectars at 30, 40 and 50% of pulp. After their preparation, stability of the nectars with the highest acceptability scores was studied during refrigerated storage. The study revealed that the pulp of Amelie is richer in water (83.34 ± 0.32%), titrable acidity (26.10 ± 0.00 meq/100g), vitamin C (122.44 ± 19.91 mg/100g), total polyphenols (35.46 ± 2.73 mg/100g) and tannins (28.89 mg/100g). In contrast, dry extract (18°Brix), pH (4.12 ± 0.01), protein (2.16 ± 0.21%), total sugars (10.41 ± 0.28 mg/100g), oxalate (164 ± 2.00 mg/100 g) and mineral elements had the highest values in Kent pulp. For nectars, the formula FK1, FK2 and FK3 with respectively 30, 40 and 50% of pulps form Kent variety recorded the highest pH (3.30 ± 0.01), titrable acidity (100.33 meq/100ml) and protein content (1.10±0.04). The formula FK3 had the highest total polyphenol content (32.06 ± 0.03 mg/100g). In contrast, the formula FA1 and FA3 of nectars with respectively 30 and 50% of pulps from Amelie variety had the highest levels of total sugar (14.33 0.67%) and vitamin C (65.12 ± 2.48 mg/100g). In addition, nectars FA3 and FK3 showed the best acceptability ratings with respective scores of 7 and 7.5. After four (04) weeks of refrigeration, the formula FA3 revealed the highest titrable acidity values (118.00±0.50) and polyphenol losses (43.18%). However, the formula F
Keywords
Mango varieties, Upgrading; Amelie, Kent, Nectars, Acceptability, Korhogo
Article Details
1. Introduction
The mango (Mangifera indica) is a fruit widely produced in the tropics and subtropics [1]. The world production of this fruit was estimated at around 48.3613 million tons during 2017. The mango is the fifth fruit production in world production after citrus, grapes, bananas and apples [2]. In 2017, the production of mango from Côte d’Ivoire was 150.000 tons with 32.400 tons (21.6%) exported. However, for the same year, local consumption of mango was estimated at 50,000 tons, about 33.33% of the production [3]. The mango is a fruit with a high nutritional value and especially appreciated for its richness in antioxidants including around 16% of vitamin C [4]. This richness in antioxidants gives it several therapeutic properties [5]. In addition, mango fibers help to reduce the blood cholesterol, thus reducing the risk of accidents and cardiovascular diseases [6]. Mango nutritional qualities, more particularly contents of vitamin C, vitamin A and fiber constitute a considerable food complements [7]. In addition, its regular consumption could also constitute an effective means of combating vitamin A deficiency [1]. Despite the nutritional and economic importance, mango use is limited because its perisability resulting in enormous post-harvest losses [1]. These post-harvest losses are around 80% per year worldwide [8]. In 2017, the losses of mango in Côte d’Ivoire were estimated at around 45% of the production [3]. This constitutes a shortfall for producers and exporters of mango. The valorization of upgraded mango could be a promising solution that would reduce these losses. Thus, it could be help to fight against food insecurity, improve the income of farmers and exporters with the creation of employment. Therefore, the present study aimed to produce nectars with pulps of the upgraded mango varieties namely Amelie and Kent from SODIPEX-SARL, an exporting company of Korhogo (Northern Côte d’Ivoire).
2. Materials and Methods
2.1 Biological materials
The biological material consists of upgraded mango varieties of Kent and Amelie from the SODIPEX-SARL company located at Korhogo (Northern Côte d’Ivoire).
Production of mango pulps
The pulp of each variety of mango was obtained according to the method described by FIRCA [9] modified. The two upgrade mango varieties collected form SODIPEX-SARL compagny were washed at the Laboratory of University Peleforo Gon Coulibaly (Korhogo, Northern Côte d’Ivoire). After washing, they were disinfected in water containing chlorine at 100 ppm. The mangoes were then peeled and their pulp separated from the kernel. The obtained was crushed using a blender (Nasco, South Korea). The crushed pulps of each mango varieties (Amelie and Kent) were stored in bottles and keep in refrigerator for further study.
Formulation and production of nectars
The production of the different nectars was carried out according to the method described by FIRCA [9]. The formulations of the nectars were made at concentrations of 30, 40 and 50% of crushed pulp added to potable water. The quantity of sugar was added to each formulation of nectar at a refractometric solids content of 20°Brix. The nectars obtained were pasteurized at 80°C during 10 minutes in a water bath. After cooling, the nectars were stored in plastic bottles. The different formulations are consigned in Table 1.
|
Nectars of Amelie |
Nectars of Kent |
|||||
|
Constituants |
FA1 |
FA2 |
FA3 |
FK1 |
FK2 |
FK3 |
|
Crushed pulp % |
30 |
40 |
50 |
30 |
40 |
50 |
|
Water % |
70 |
60 |
50 |
70 |
60 |
50 |
|
Sugar (g) |
212.5 |
181 |
150 |
375 |
262.5 |
225 |
|
Lemon juice (mL) |
25 |
25 |
25 |
25 |
25 |
25 |
Table 1: Composition of mango nectar formulations
2.2 Physicochemical analysis
Moisture, proteins, pH and and acidity were determined using official methods of AOAC [10]. pH was determined as follow: 10 g of crushed pulp was homogenized with 100 mL of distilled water and then filtered through Whatman No. 4 filter paper. The pH value was recorded after the electrode of pH-meter (Hanna, Spain) was immersed into the filtered solution and nectar. For acidity 10 mL of filtrate or nectar have been titrated by NaOH 0.1N. The moisture content was determined by the difference of weight before and after drying pulp (10 g) in an oven (Memmert, Germany) at 105°C until constant weight. The soluble dry extract of the pulps and nectars were measured using a refractometer. A drop of crushed pulp or nectar is placed on the screen of refractometer and the reading is taken directly.
2.3 Nutritive properties
The total sugars were determinited by the phenol method [11]. 0.50 g of crushed pulp or 0.5 mL of nectar was introduced into a test tube containing 0.50 mL of sulfuric acid (12 N). The reaction medium was keep at ambient temperature (25 °C) during 1 hour before boiling it for two hours in a water bath (100 °C). After boiling, were added to the contents of the tube successively, 5.50 mL of distilled water, 10 mL of ethanol (70%), 0.5 mL of zinc sulfate (2 g / 100 mL) and 0.5 mL of potassium ferrocyanide (10.6 g / 100 mL). The mixture was filtered and the filtrate was adjusted to 50 mL with distilled water. To 0.2 mL of the filtrate was successively added 0.50 mL of phenol (5%) and 2.50 mL of sulfuric acid. After for 10 minutes at ambient temperature, the mixture is well homogenized and the absorbance was read with a spectrophotometer at 490 nm. The total sugars content was determined using a calibration curve of glucose (10 mg / 100 mL) as standard. Proteins were determined through the Kjeldhal method. Vitamin C contained in analyzed samples was determined by titration using the Method described by [12]. About 10 g of pulp or 10 mL of nectar were soaked for 10 min in 40 mL metaphosphoricacid-acetic acid (2%, w/v). The mixture was centrifuged at 3000 rpm for 20 min and the supernatant obtained was diluted and adjusted with 50 mL of bi-distilled water. Ten (10) mL of this mixture was titrated to the end point with dichlorophenol-indophenol (DCPIP) 0.5 g/L. Polyphenols content was determined using the method reported by [13]. A quantity of 1 g of crushed pulp or 5 mL of nectar was soaked in 10 mL of methanol (70%) and centrifuged at 1000 rpm for 10 min. An aliquot (1 mL) of supernatant was oxidized with 1 mL of Folin-Ciocalteu’s reagent and neutralized by 1 mL of sodium carbonate (20%). The reaction mixture was incubated for 30 min at ambient temperature and absorbance was measured at 745 nm by using a spectrophotometer. The polyphenols content was obtained using a calibration curve of gallic acid (1 mg/mL) as standard. The minerals contents in the pulp was carried out according to the method described by CEAEQ [14]. 5g of pulp were burned to ashes in a muffle furnace. The ashes obtained were dissolved in 10 mL of HCl/HNO3, transferred into 100 mL flasks and the volume was made up using deionized water. The mineral composition of each sample was determined using an agilent 7500c inductively coupled argon plasma mass spectrometer (ICP-MS). Calibrations were performed using external standards prepared from a 1000 ppm single stock solution made up with 2% nitric acid.
2.4 Anti-nutritional factors
Oxalates content was performed using a titration method [15]. One (1) g of dried powdered sample was weighed into 100 mL conical flask. A quantity of 75 mL of sulphuric acid (3 M) was added and stirred for 1 hour with a magnetic stirrer. The mixture was filtered and 25 mL of the filtrate was titrated while hot against KMnO4 solution (0.05 M) to the end point. Tannins of samples were quantified according to [16]. For this, 1mL ofthe methanolic extract was mixed with 5 mL of vanillin reagent and the mixture was allowed to incubate at ambient temperature for 30 min. There after, the absorbance was read at 500 nm by using a spectrophotometer. Tannins content of samples was estimated using a calibration curve of tannic acid (2 mg/mL) as standard.
2.5 Sensory analysis
The sensory characterization of the nectars was carried out through a hedonic test with ten (10) untrained subjects [17]. The test is based on the preference of the panelists in terms of appearance, color, acidity and flavor on a scale from 1 to 9 points which indicates the level of panelist preference. The nectars are then coded according to the pulp concentration (30.40 and 50%) and submitted to each panelist randomly, respecting the increasing order of pulp concentration.
2.6 Statistical analysis
The statistical analyses were performed with Graph Pad Prism software version 8.0.2 (263). The variance analysis (ANOVA) was performed to determine differences between the averages according to method of Turkey at the 5% threshold (p<0.05 was considered significant). The results were expressed as averages with standard error on mean (mean ± SEM).
3. Results and Discussion
Proximate composition of the selected pulp is presented in Table 2. The physicochemical parameters generally differ significantly (p<0.05) from a pulp to another. The Amelie variety revealed the highest moisture value (83.34 ± 0.32%) compared to the Kent variety (77.34 ± 0.48%). These moistures values were lower than those obtained by Collin and Dainic [18] with Amelie (85.63%) and Kent (79.53%) varieties in Korhogo during the 1989 mango season. The high moisture content of Amelie pulp could make it susceptible to dehydration and the proliferation of microorganisms, which could impact its shelf life, hence the need to store it under adequate conditions. Moreover, this high moisture content could constitute an advantage for the use of these mangos as a diuretic and invigorating on the medicinal level [19]. Pulp of Kent variety recorded the highest pH value (4.12 ± 0.01) while the acidity was higher in Amelie pulp (26.10 ± 0.00 meq / 100g). The pH of the pulps studied were more or less higher than those of Traoré [7] which obtained values of 3.71 and 3.93 respectively for the Amelie and Kent varieties. The acidities obtained were approximately in agreement with those of Briot [20] which are 26.80 meq/100 g and 15.60 meq/100 g respectively for Amelie and Kent in Korhogo. The high acidity of the Amelie variety is believed to be due to the presence of various organic acids. Therefore, the aqueous extract of this variety could be exploited as a conservative in food industry [21]. The dry extract of the pulps ranges from 17 to 18 °Brix. Kent has the highest value. The values of the varieties studied differ from that of Guepratte [22] which recorded values of 19.6 and 13.9 °Brix respectively for Kent and Amelie. According to Bissardon [23] the soluble dry extract gradually increases with the degree of maturity. The Kent variety would contain more sugars.
|
Parameters |
Amelie |
Kent |
|
Monsture (%) |
83.34 ± 0.32a |
77.34±0.48b |
|
Dry extract (°Brix) |
17.00 ± 0.00b |
18.00 ± 0.00a |
|
pH |
3.30 ± 0.00a |
4.12 ± 0.01b |
|
Acidity (meq/100g) |
26.10 ± 0.00a |
15.05 ± 0.05b |
Table 2: Physicochemical characteristics of mango pulps Amelie and Kent
Data are represented as Means ± SD (n = 3).
Means in the column with no common letter differ significantly (p<0.05)
Nutritive properties of the selected pulp are in Table 3. There are a significant difference (p<0.05) between most of these parameters. Protein content of Kent (2.16 ± 0.21%) was high compared to Amélie (1.34 ± 0.04%). The protein contents of the varieties studied are higher than those of Traoré [7], whose values were 0.63 and 0.61% respectively for Amelie and Kent. Being total sugars, they are statistically different (p <0.05). Kent recorded the high total sugar content (10.41 ± 0.28 mg/100g) contrary to Amelie (8.31 ± 0.08 mg/100g), which correlates with the dry extract. The sugar content of the Kent variety studied differs from that of Djantou [24] which recorded a value of 16.39% in the same variety in Cameroon. This low level of total sugars could be explained by the stage of maturity of the mangos because during maturation the starch is hydrolyzed into glucose which is isomerized into fructose and sucrose [25]. They are statistically different between the vitamin C and polyphenols content of pulps, The contents of vitamin C were 111.72 ± 1.44 mg/100g for Kent and 122.44 ± 1.15 mg/100g for Amelie. Polyphenols contents of pulps were 32.63 ± 1.62 mg/100g for Kent and 35.46 ± 2.73 mg/100g for Amelie. The Amelie variety is characterized by the highest values of vitamin C and polyphenols. Vitamin C is recognized for its antioxidant properties which protect cells against free radicals and its role in iron absorption [5, 21]. The vitamin C contents of the varieties studied were higher than those of [5] who recorded values ranging from 19.79 to 34.59 mg/100 g in eight varieties of mango in China. These values were also higher than those of Sawadogi-Lingani et Traoré [26] for the Amelie variety (49.50 mg/100 g) from the Zoula zone in Burkina-Faso. This high content could due to at the stage of maturity of mangos because the vitamin C decreases during maturity and post-harvest treatment in fruits [27]. However, the mangos studied could meet the daily requirement of vitamin C which is 40 mg [28]. Phenolic compound, called secondary metabolites, belong principal antioxidants in plants, alongside vitamin C, vitamin E and carotenoids. The Amelie variety with high percentages of polyphenols could be recommended for the prevention of cardiovascular diseases. The mineral element contents of the pulps studied were statistically different (p<0.05). The content of calcium, phosphorus, magnesium and iron were higher in the pulp of the Kent variety with the respective contents 19.60 ± 0.00 ; 15.57 ± 0.10 and 14.92 ± 0.00 mg/100 g and 0.28 ± 0.04 mg/100g. The calcium and magnesium contents of the varieties studied were higher than those (13 mg/100g) determined by Traoré [7]. Calcium and magnesium are minerals contribute to control the blood pressure [29]. The phosphorus contents of the mangos studied were much lower than those (16 mg/100g) determined by Traoré [7]. This mineral is essential in the formation of bones, vitamin D absorption and cellular energy production [30].
|
Parameters |
Amelie |
Kent |
|
Total sugars (%) |
8.31 ± 0.08b |
10.41 ± 0.28a |
|
Proteins (%) |
1.34 ± 0.04b |
2.16 ± 0.21a |
|
Vitamin C mg/100g |
122.44 ± 1.44a |
111.72 ± 1.15b |
|
Polyphenols mg/100g |
35.46 ± 1.05a |
32.63 ± 0.94b |
|
Calcium (Ca) |
15.21 ± 0.60b |
19.60 ± 0.00a |
|
Magnesium (Mg) |
9.84 ± 0.33b |
14.92 ± 0.00a |
|
Iron (Fe) |
0.13 ± 0.01b |
0.28 ± 0.04a |
|
Phosphorus (P) |
10.73 ± 0.30b |
15.57 ± 0.10a |
Table 3: Nutritive properties of pulps.
Data are represented as Means ± SD (n = 3). Means in the column with no common letter differ significantly (p<0.05)
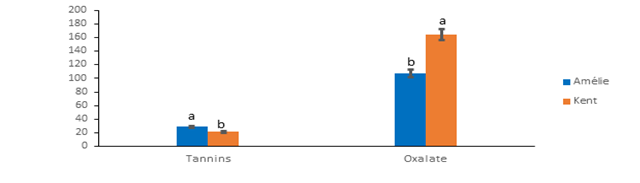
The levels of anti-nutrient factors of the mango pulps are on Figure 1. Kent recorded the highest oxalate content (164 ± 2.00 mg/100g) compared to Amelie (107.33 ± 1.15 mg/100g). Oxalates constitute anti-nutritional factors which interfere in the biodisponibility of important minerals such as calcium, magnesium, zinc and iron [31]. The varieties studied have contents below the organism's oxalate tolerance zone, which is between 200 and 500 mg/100 g [32]. The consumption of the mangos studied could not influence the biodisponibility of minerals. Contrary to oxalates, Amelie recorded the highest tannin content (28.89 ± 0.85 mg/100g) compared to Kent (21.85 ± 1.05 mg/100g). Tannins have an inhibitory activity on digestive enzymes while being complexed with proteins [33]. This inhibitory action would be marked when the protein content is lower than 10% [34]. The Amelie and Kent varieties with respective protein contents of 1.34% and 2.16% could affect the biodisponibility of digestive enzymes.
The physicochemical properties of the nectars produced are in Table 4. The pH of nectars form Amelie pulps were statistically identical with an average value of 3.26. For Kent nectars the pH values were between 3.18 ± 0.00 and 3.30 ± 0.01 with an average of 3.23. The formulation FK2 recorded the highest value. These values were lower than that obtained by Mahfouf and Ihedaden [35] which was 3.83. But those obtained by Djoudi and Zitouni [36] and Cissé [37] with respectively Kent nectar (3.22) and Baobab nectar (3 to 3.25) were agree with these pH values. This distinction can be due to the difference of the selected fruit or to the addition of lemon juice during formulation in order to create an unfavorable environment for the development of microorganisms. This acidic pH helps preserve the drink against microbiological alterations [38]. Sugars are the constituents that determine the sweetness of a food. Moreover, they play an essential role in the conservation of food products, the osmotic pressure which they exert on microorganisms and the lowering of the water activity of the food [39]. The average total sugar content of our elaborate nectars was in the range of 11.34 to 14.33 g/100mL. These levels agree with those of Akkouche and Chikhaoui [19] with values varying from 5.28 to 14.51 g/100mL in melon nectar and tangerine juice.
|
Nectars |
Formulations |
pH |
Acidity (meq/100 mL) |
Total sugars (g/100 mL) |
|
Amelie |
FA1 (30%) |
3.26 ± 0.01c |
82.33 ± 1.08b |
14.33 ± 0.67a |
|
FA2 (40%) |
3.27 ± 0.01c |
82.23 ± 0.10b |
11.95 ± 0.12b |
|
|
FA3 (50%) |
3.25 ± 0.01c |
82.33 ± 0.68b |
10.19 ± 0.08c |
|
|
Kent |
FK1 (30%) |
3.18 ± 0.01a |
100.33 ± 2.88a |
13.72 ± 0.05a |
|
FK2 (40%) |
3.30 ± 0.01d |
80.66 ± 2.88b |
11.55 ± 0.36b |
|
|
FK3 (50%) |
3.21 ± 0.01b |
83.33 ± 2.88b |
11.34 ± 0.02b |
Data are represented as Means ± SD (n = 3). Means in the column with no common letter differ significantly (p<0.05)
Table 4: Physicochemical properties of nectars
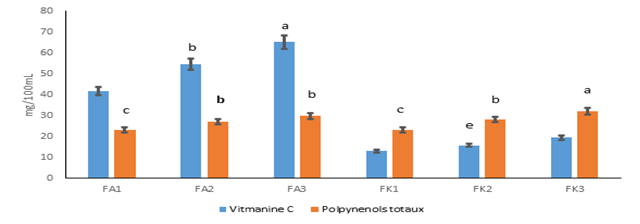
Figure 2 presents the contents of vitamin C and total polyphenols. Vitamin C contents varied from 12.90 to 65.12 mg/100 mL. Amelie nectars recorded the highest levels of vitamin C with the formulation FA3 (65.12 mg/100 mL). For Kent nectars, they recorded the lowest levels with the formulation FK1 (12.90 mg/100 mL). The vitamin C contents of Kent nectars varied from 12.9 ± 1.00 to 19.36 ± 0.00 mg/100mL were agree with that of melon nectar inclued between 4.287 and 40.6 mg/100mL [40]. These values were lower than the vitamin C levels of Amelie nectars (41.65 ± 2.68 to 65.12 ± 2.48 mg/100mL). This variation of the vitamin C contents of different nectars studied could be explained by the reaction speed of the oxidation of vitamin C, which would increase with heat during pasteurization [41]. However, the consumption of 100 mL of the various Amelie nectars could cover the daily vitamin C requirements which are estimated at 40 mg [28]. Concerning the total polyphenols, the levels were between 23.06 and 32.07 mg/100 mL. The formulation FK3 recorded the highest content (32.07 mg/100 mL). The lowest content was obtained in the formulations FA1 and FK1. The other formulations have statistically identical contents. Polyphenol contents of formulated nectars were much higher than those of melon nectar and tangerine juice (0.047 and 0.082 mg/100 mL) studied by Akkouche and Chikhaoui [19]. This difference could be explained by the heat treatment applied during the production and the action of polyphenol oxidases during the embrittlement of cellar membranes. According to Iannou and Ghoul [42], pasteurization induces losses of phenolic compound.
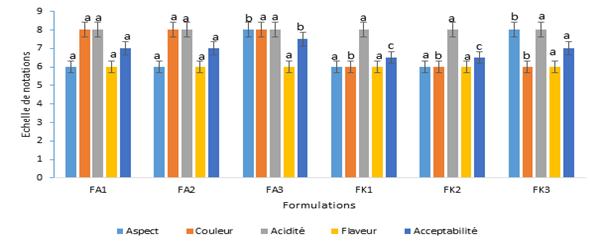
Sensory characteristics of different nectar formulations are presented by Figure 3. The formulations of nectars FA3 and FK3 nectars revelead the highest score (8) in terms of appearance. However, the color of Amelie nectars was more appreciated by the panelists with a score of (8) compared to that of the Kent variety (6). The flavor and the acidity were similar for the nectars of the two varieties with respective scores of 6 and 8. Concerning the acceptability criterion, the formulations of nectars FA3 and FK3 recorded the best scores with respective values of 7.5 and 7.
Conclusion
The results obtained at the end of our work make it possible to pronounce us on the food potentialities of non-exportable mangos. Mango pulps are considerable sources out of total sugars, vitamin C, polyphenols and minerals that could be valued for food. The nectars produced contain appreciable quantities of vitamin C and polyphenols. The formulations FA3 and FK3 were appreciated nectars. All these proven food potentialities of non-exportable mangos could be exploited to reduce losses through their transformation into nectar. To ensure nutritional balance, it would be desirable to associate the Kent and Amelie varieties in the production of mango nectars (cocktail). The study showed that the storage at 4 °C of nectars produced could be considered. This study reveals that the valorization of the upgraded mango of exporting compagnies in Northern Côte d’Ivoire could contribute to produce nectars with high nutritional value. Finally, production of nectars from mango could be effectively fight against food insecurity and certainly reduce the post-harvest losses to improve the income of farmers and exporters.
Acknowledgement
The authors would like to express their gratitude to the SODIPEX Company (Korhogo, Ivory Coast) for its collaboration in the collection of samples of mango varieties used in this research work.
References
- Baudelaire E, Njantou D. Optimisation du broyage des mangues séchées (Manguifera indica var Kent) : influence sur les propriétés physicochimiques et fonctionnelles des poudres obtenues. Vandœuvre-lès-Nancy, INPL, (2006).
- FAO Stat. Major tropical fruits. Statistical compendium 2017, Rome, (2019): 38.
- Répertoire de technologies et procédés de transformation de la mangue et de l’ananas. (2019): 120.
- De La, Cruz MJ, Garcia HS. Mango: Post-harvest Operations. AGSI/FAO: Danilo Mejia, PhD (Technical), Beverly Lewis (Language & Style). Last reviewed (2002): 70.
- Ma X, Wu H, Liu L, Yao Q, Wang S, et al. Polyphenolic compounds and antioxidant properties in mango fruits. Scientia Horticulturae 129 (2011): 102-107.
- Rivard-Gervais N. Aliments fonctionnels et produits nutraceutiques: les fibres, les vitamines et les autres éléments nutritifs. Le Médecin du Québec 36 (2001): 61-68.
- Traoré HK. Valorisation des variétés de mangue produite au Burkina Faso : Aspects biochimiques, biotechnologies et nutritionnelles. Thèse de doctorat unique. Université Joseph Ki-Zerbo de Ouagadougou. (2019): 28-70.
- Kansci G, Koubala B, Mbome L. Effect of rippeneing on the composition and suitability for jam processing of different varieties of mango (Mangifera Indica). African Journal of Biotechnology. 2 (2003): 301-306.
- Répertoire de technologies et procédés de transformation de la mangue et de l’ananas. (2014): 20.
- Official methods of analysis of the association of official analytical Chemists: 981.12 Arlington, USA. 15 (1990): 910-928.
- Dubois M, Gilles AK, Hamilton JK, Ribers PA. Colorimetric method for determination of sugars related substances. Chemistry 28 (1956): 350-356.
- Pongracz G, Weiser H, Matzinger D. Tocopherols-Antioxydant. Fat Science Press, Washington DC. (1971): 64.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteureagent. Meth Enzymol 299 (1999): 152-226.
- Mineral determination. Argon plasma spectrometry method, MA 200, Met 1.2, Rev 4. Quebec, Canada (2013): 24.
- Day RA Underwood AL. Quantitative analysis. (5th edtn), Prentice Hall. (1986): 701.
- Bainbridge ZK, Tomlins, Westby. Analysis of condensed tannins using acidified vanillin. J. Food Sci. Agric. 29 (1996): 77-79.
- Cheftel JC, Cheftel H. Introduction à la biochimie et à la technologie des aliments. Techniques et documentation, 3 ème édition. Lavoisier. Paris, France (1980): 381.
- Collin MN, Dalnic R. Comparaison de mangues en provenance de Côte d’Ivoire. Journ. Agrumes/mangues, Irfa, Inra, Montpellier, France. (1991): 50.
- Akkouche T, Chikhaoui K. Caractérisation d’une variété de melon (Cucumis melo-L) et essai de préparation de boissons nectars à base de deux fruits (melon et mandarine). Mémoire de Master. Université Mouloud Mammeri de Tizi-Ouzou. Alger (2018): 38-39.
- Briot E. Étude de la physiologie post récolte de la mangue: projet de fin d’études. UTC de Compiègne, France (1999): 55.
- Alais C, Linden G. Abrégé de biochimie alimentaire. Masson ed., Paris (1997): 248.
- Guépratte M. Physiologie post récolte de la mangue et conservation sous atmosphère modifiée. Mémoire de fin d’étude, École supérieure d’agriculture d’Angers (ESA), Angers. (1998): 44.
- Bissardon F. Physiologie post récolte de la mangue et conservation en froid de la mangue. Mém. ingénieur Ensia, Montpellier, France. (1999): 21.
- Djantou NEB Optimisation du broyage des mangues séchées (Manguifera indica var Kent) : influence sur les propriétés physicochimiques et fonctionnelles des poudres obtenues. Thèse de Doctorat, Institut National Polytechnique de Lorraine, France (2006): 126.
- Takuji I, Katsuaki S, Yasuji Y. Changes in respiration rate, saccharide and organic acid content during ripening of mango fruit (Mangifera Indica L. ‘Irwin’) cultured in a plastic house. J. Japan.Soc. Hort. Hort. Sci. 66 (1997): 629-635.
- Sawadogo-Lingani H, Traore A. Composition chimique et valeur nutritive de la mangue Amélie (Mangifera indica L.) du Burkina Faso. Journal des Sciences. 2 (2001): 35-39.
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology. 20 (2000): 207-220.
- Human vitamin and mineral requirements. FAO edtn. (2004): 361.
- Ranhotra GS, Gelroth JA, Leinen SO, Vmasand MA, Lorciz KJ. Nutritional profile of some edible plants from Mexico. J. Food Comp. Anal. 11 (1998): 298-304.
- Depezay L. Les légumes dans l’alimentation: leurs effets nutritionnels. Fondation Louis Bonduelle edtn, France. (2006): 7.
- Hassan LG, Umar KJ, Dangoggo SM, Maigandi AS. Antinutrient composition and bioavailability prediction as exemplified by calcium, iron and zinc in Melocia corchorifolia leaves. Pakistan Journal of Nutrition. 10 (2011): 23-24.
- Munro A, Bassir O. Oxalates in Nigerian vegetables. W. Afri. Bio. Appl. Chem. 12 (1969): 14-18.
- Carnovale E, Lugaro E, Marconi E. Protein quality and antinutritional factors in wild and cultivated species of Vigna spp. Plant Food Hum. Nutr. 41 (1991): 11-20.
- Li J, Liu J, Tao S. Effects of tannic acid on the food intake and protein digestibility of root voles. Acta Theriologica Sinica. 23 (2003): 52-57.
- Mahfouf T, Ihedaden L. Essais de fabrication d’un nectar de melon (Cucumis melo L.) et étude de la stabilité. Diplôme de Master 2. Université Mouloud Mammeri, Tizi Ouzou. 106 (2017).
- Djoudi F, Zitouni S. Formulation d’une boisson à base de purée de tomate, de fraise et de raisin rouge. Mémoire d’ingénieur, INA, Alger, (2010): 11-70.
- Cissé I. Caractérisation des propriétés biochimiques et nutritionnelles de la pulpe de baobab des espèces endémiques de Madagascar et d'Afrique continentale en vue de leur valorisation. Mémoire de Thèse de Doctorat. Centre international d'études supérieures en sciences agronomiques, Montpellier SupAgro. Montpellier, France. (2012): 153.
- Benaissa A. Etude de la qualité microbiologique des viandes cameline et ovine conservées selon différents modes. Mémoire de Master, Université Kasdi Merbah Ouargla, Algérie. (2011): 65.
- Achir Z, Hammar L. Caractérisation physico-chimique des Mûres (Rubusfructicosus) et essai de fabrication d’une boisson SMOOTHIES. Mémoire d’ingénieur. Université Mouloud Mammeri de Tizi-ouzou, Alger, (2010): 36.
- Abas S, Talbi M, Cité par Akkouche T, Chikhaoui K. Caractérisation d’une variété de melon (Cucumis melo-L) et essai de préparation de boissons nectars à base de deux fruits (melon et mandarine). Mémoire de Master. Université Mouloud Mammeri de Tizi-Ouzou, Alger. (2018): 38-39.
- Bourgeois CM, Mesclej F, Zucca J. Microbiologie alimentaire, aspect microbiologique de la sécurité alimentaire, Tome 1. ed., Lavoisier Tec et Doc, Paris, France (1996): 672.
- Ioannou I, Ghoul M. Biological activities and effects of food processing on flavonoids as phenoliques antioxydants. In : Marian P. Advances in Applied Biotechnology. (2012): 101-124.


 Impact Factor: * 3.8
Impact Factor: * 3.8 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 